Thymectomy versus tumor resection for early-stage thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis
Introduction
Thymic epithelial tumors (TETs) should be resected together with the surrounding thymus and fatty tissue rather than shelled out because all TETs are considered malignant and transcapsular invasion is difficult to detect intraoperatively (1). However, there is no consensus on the appropriate extent of thymic resection (2-4). Although thymectomy may help prevent potential risks of postoperative myasthenia gravis (MG) and intrathymic or locoregional recurrence (5,6), some consider that tumor resection alone (thymomectomy) may be enough for noninvasive thymomas without MG (2,3). Also, increased use of CT screening for lung cancer has led to more cases of incidentally detected small lesions at early stage. And advent of minimally invasive surgery in recent years also contributed to increased interest in video-assisted thoracoscopic surgery (VATS) thymomectomy (7). Although some single-center studies have shown no statistical differences in disease-free survival between thymectomy and thymomectomy, the follow-up durations were relatively short despite of the rarity and the indolent nature of thymomas (2,3). Thus, it is important to compare long-term outcomes on a larger patient population base so as to determine the appropriate extent of resection for the disease.
The objective of this study was to evaluate the surgical outcomes of tumor resection with or without total thymectomy for TETs using a retrospective database of thymoma cases constructed by the Chinese Alliance for Research in Thymomas (ChART).
Materials and methods
The ChART, initiated by 18 tertiary referral centers in China, retrospectively collected the clinical data of 2,104 patients with thymic tumors from 1994 to 2012. The present study enrolled only 1,047 patients with early-stage tumors (Masaoka-Koga stage I and II) with no pretreatment. The study was proved by the hospital IRB. The following clinical data were collected: general information, presence of MG and other autoimmune diseases, surgical approach, postoperative histological type, postoperative clinic-pathological stage, and follow-up data. Because only de-identified data were used for the study, informed consent was waived by IRB. Preoperative classification and surgical treatment evaluation were performed in patients with MG according to both the Myasthenia Gravis Foundation of America Clinical Classification and Post-Intervention Status (8). Histological typing of tumors was classified according to the World Health Organization 2004 Classification of Thymoma. Clinic-pathological staging was performed according to the Masaoka-Koga staging system (9).
Surgical approaches included sternotomy, thoracotomy, and video-assisted thoracoscopic surgery (VATS). In this multi-center retrospective study, there was no uniform standard for the selection of the surgical approach; the surgeons chose the approach according to their preference. Likewise, there was no uniform standard for the selection of adjuvant therapy among the patients; decisions on adjuvant therapy were mostly based on the physicians’ subjective evaluation.
Patients were divided into thymectomy and thymomectomy groups based on the resection extent of the thymus. In the thymectomy group, 796 patients underwent total or subtotal thymectomy to remove all thymic tissue, including the anterior mediastinal fat, on the basis of complete tumor resection. In the thymomectomy group, 251 patients underwent complete tumor resection, including some surrounding thymic tissue, or resection of the ipsilateral lobe of the thymus.
The follow-up completed in October 2013, with a median follow-up time of 38 months, and the follow-up rate was 78.4%.
Statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Patients’ characteristics were compared between the two groups using the t-test and χ2 test. Survival analysis was performed using the Kaplan-Meier method and log-rank test. Differences were considered statistically significant when P<0.05.
Results
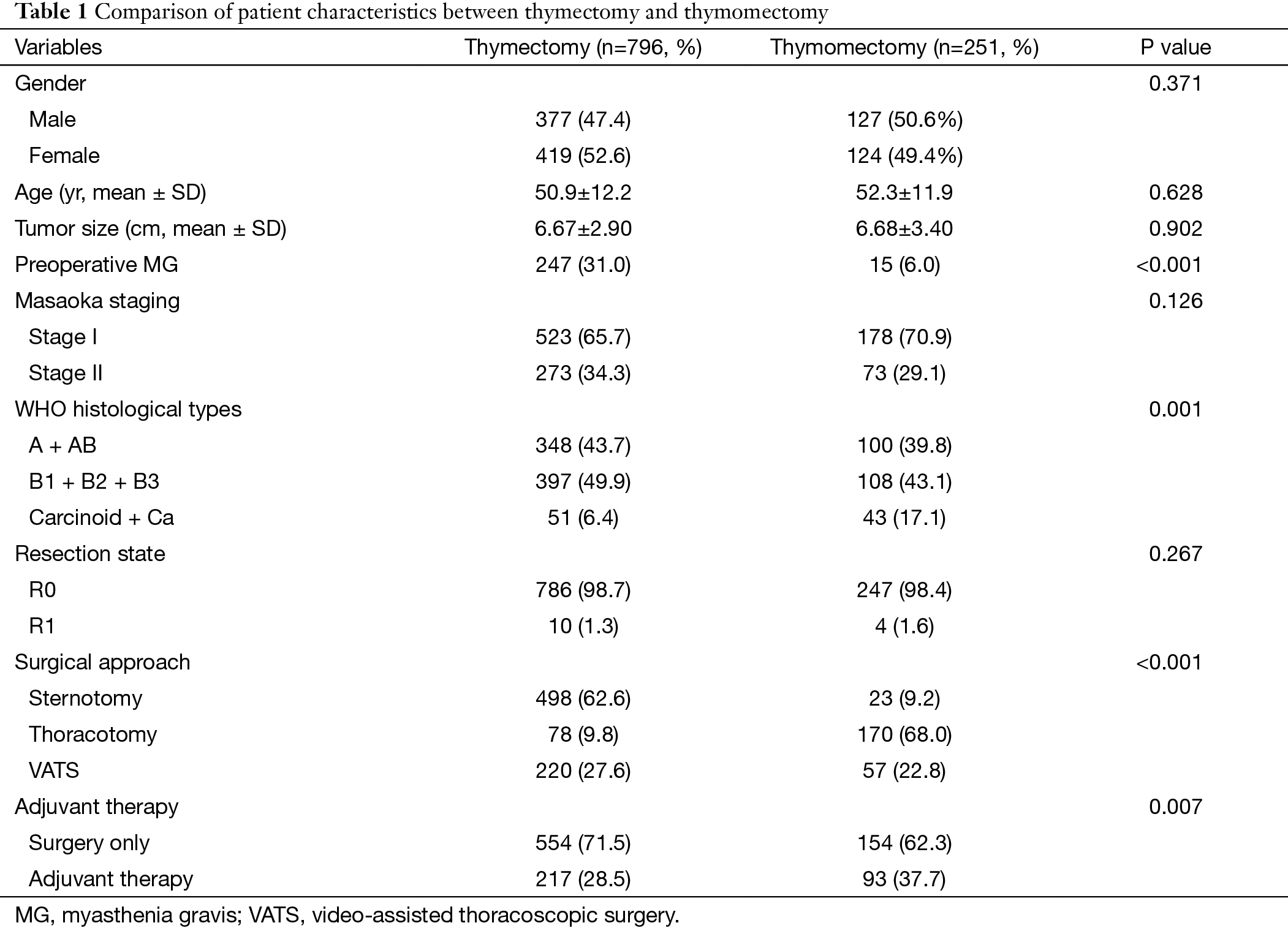
There were 504 male and 543 female in this series, with an average age of 51.3±12.1 (range, 15–83) years. A total of 1,033 patients (98.7%) underwent complete tumor resection (R0), with only 14 (1.1%) had microscopic residual disease (R1). A total of 310 patients (29.6%) received postoperative adjuvant therapies (radiotherapy and/or chemotherapy). Patients’ characteristics are shown in Table 1. No significant differences were observed in gender, age, or tumor size between the two groups. There were more stage I tumors in the thymomectomy group (70.9%) than in the thymectomy group (65.7%), but without statistical significant difference (P=0.126). However, significantly higher proportion of thymic carcinoma was seen in the thymomectomy group than in the thymectomy group (17.1% vs. 6.4%, P=0.007).
Full table
In terms of surgical approach, sternotomy was mainly used in the thymectomy group and thoracotomy was more frequently chosen in the thymomectomy group, with a significant difference (P<0.001). There was no significant difference in minimally invasive approach. And complete resection rate (R0) between the two groups were also similar (P=0.267). However, a higher proportion of patients received adjuvant therapy after thymomectomy than after thymectomy (37.7% vs. 28.5%, P=0.007).
In total, 262 patients had MG before surgery. The majority of them (247, 94%) underwent thymectomy and only 15 patients (6%) underwent thymomectomy. The proportion of patients with MG was significantly different between the two groups (P<0.001). MG remission rate was significantly higher after thymectomy than after thymomectomy (91.6% vs. 50.0%, respectively, P<0.001). Postoperative MG was found in only two patients (0.81%), both in the thymectomy group.
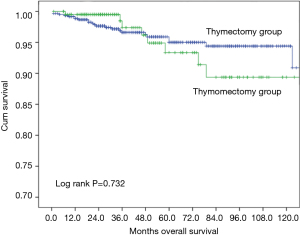
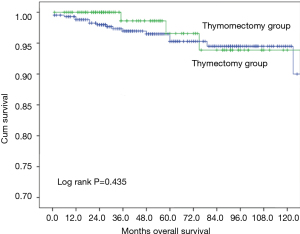
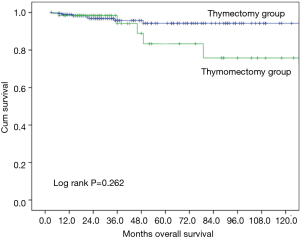
Ten-year overall survival was similar between the two groups (Figure 1, 90.9% after thymectomy and 89.4% after thymomectomy, P=0.732). Stratified analysis by Masaoka-Koga stage also did not show any significant difference in overall survival between the thymectomy and thymomectomy groups (Figures 2,3). Recurrence rate was 3.1% after thymectomy and 5.4% after thymomectomy, with no significant difference between the two groups (P=0.149). Stratified analysis did not find any significant difference in recurrence rates in Masaoka-Koga stage I tumors (3.2% vs. 1.4%, P=0.259). However in patients with Masaoka-Koga stage II tumors, recurrence was significantly less after thymectomy than after thymomectomy group (2.9% vs. 14.5%, P=0.001)
Discussion
Thymectomy through a median sternotomy has long been the gold standard for surgical treatment of TETs. In particular, when patients have concomitant autoimmune diseases such as MG before surgery, it is deemed necessary to remove all mediastinal fat on both sides during thymectomy (1). However, surgery through a median sternotomy causes marked injury, and there is a 1% to 5% risk of mediastinal infection after surgery (4). To reduce the surgical trauma, many surgeons choose to operate through intercostal thoracotomy, VATS, or transcervical incision (5-7). Although these approaches may enable resection of early-stage thymic tumors, it is technically demanding to perform a total thymectomy especially in case of intercostal thoracotomy. With improvements in imaging and surgical techniques, a growing number of small-diameter thymomas have been detected incidentally and resected through various minimally invasive approaches, especially VATS, in the clinical setting. And some surgeons tend to perform thymomectomy under VATS to avoid the risk of bleeding from the brachiocephalic vein (3). Therefore, it is necessary to evaluate the effect of the extent of thymectomy on the prognosis of early-stage TETs.
Prognosis of TETs is closely associated with tumor stage, histological type, completeness of surgical resection, and effective adjuvant therapy (10). Early-stage thymic tumors have good prognosis with low recurrence and mortality rates (11). In the current study, 10-year overall survival was as high as 90% for stage I and II tumors. Several studies comparing the extent of thymectomy for thymomas found no significant difference in postoperative recurrence rate or survival between thymectomy and thymomectomy (2-4). In the current study with stage I and II tumors, 10-year survivals after these two procedures were also similar (90.9% vs. 89.4%). However, this does not support the similar efficacy of thymomectomy to thymectomy. Adjuvant therapies were used more frequently after thymomectomy than after thymectomy in our patients. Besides, thymomas are relatively indolent tumors and long-term survival could still be expected even after tumor recurrence. In this concern, recurrence status is often considered a better index for evaluation of management outcome. In the current study, although postoperative recurrence rates were also similar after these two procedures (3.1% vs. 5.4%, P=0.149), stratified analysis revealed a significantly increased risk of tumor recurrence after thymomectomy in Masaoka-Koga stage II tumors (2.9% vs. 14.5%, P=0.001). Masaoka-Koga stage II refers to tumors infiltrating the thymus or surrounding adipose tissue. For well encapsulated Masaoka-Koga stage I tumors, complete tumor removal may be readily achieved by either thymomectomy or thymectomy. For Masaoka-Koga stage II tumors, it is basically impossible to determine the extent of tumor invasion during surgery. Then without an accurate judgment of tumor margin, there is potentially an increased risk of tumor spillage during thymomectomy. And tumor implantation in the pleural cavity is the most often encountered recurrence pattern in thymic tumors. This may also help explain the higher recurrence rate in the thymomectomy group for Masaoka-Koga stage II tumors in our study.
Theoretically, either thymomectomy or thymectomy can be desirable procedure for stage I tumors which are confined to a complete capsule without any invasion into the surrounding structures. And there was no significant difference between the two groups in overall survival or recurrence rates for Masaoka-Koga stage I tumors. However, it is extremely difficult to accurately define a stage I tumor before operation or during. Computed tomography (CT) is the most widely used imaging technique for the diagnosis of thymic tumors. The International Thymic Malignancy Interest Group (ITMIG) has also recommended the use of CT as a standard examination for preoperative staging (12). Yet, few studies have focused its usefulness and accuracy (13). Overall, CT scan has relatively low sensitivity and specificity for early-stage TETs, and there is no way to accurately identify Masaoka-Koga stage I tumors from stage II diseases (14). Although positron emission tomography scan may help distinguish thymomas from more malignant thymic carcinomas, its staging accuracy is not high enough in lesions without obvious invasion into the neighboring structures, especially in small-diameter tumors (15). Similar to the management for most other malignancies, the goal of surgery lies not only in complete removal but also accurate staging of the disease. Therefore even for clinically stage I tumors, thymectomy should still be recommended.
Thymectomy has been shown to be effective in treating TETs with concomitant MG before surgery, with an improvement rate of 73% to 89%, and a complete remission rate ranging from 28% to 52% (8,15-18). Studies to date mainly compared the therapeutic effects of surgery with medical treatment (cholinesterase inhibitors and immunosuppressive agents), with favorable results showing higher improvement rates in patients who received thymectomy than medical treatment (16,17). Up till now, no study has ever compared the effect of the extent of thymectomy on the outcome of surgical treatment for MG with concomitant thymomas. In the present study, most MG patients received thymectomy and only 15 patients had thymomectomy. And the postoperative improvement rate of MG was 50% in these 15 patients, far below that in patients in the thymectomy group (91.8%). This is in accordance with the reports from Sonett et al. (19) showing that increased extent of clearance of the thymus and mediastinal fat might help improve the remission rate for MG. Our finding suggests that at least for those thymoma patients concomitant with MG, thymectomy, instead of tumor resection alone, should be chosen to ensure a satisfactory outcome.
Another concern is that thymoma patients without MG before surgery still carry the risk of developing MG after tumor resection at a reported rate of 1.5% to 28.0% (5,6). Whether the extent of thymectomy affects the development of MG after surgery remains unclear. Ito et al. (18) reported that the incidence of postoperative MG was 5.0% in the thymectomy group and 4.2% in the thymomectomy group, with no significant difference between the two groups. Similar results were reported by Tseng et al. (3) and Onuki et al. (2). In the present study, only two patients (0.81%) without preoperative MG developed MG after surgery, both in the thymectomy group. This seems to indicate that postoperative MG is very rare and total thymectomy does not help prevent the risk of newly onset MG in patients without preoperative MG.
Limitations of our study include its retrospective nature and the associated inherent selection biases. Extent of resection and selection of postoperative adjuvant therapy were mostly based on the surgeons’ own preferences without uniformed standards. And the dropout rate was also relatively high. Although stratified analysis was used to rule out potential confounding biases to the greatest extent, prospective randomized controlled studies are still necessary to further validate our findings.
Conclusions
Although overall survival appeared to be similar after tumor resection alone and thymectomy, there is no sufficient evidence to support the routine application of thymomectomy for thymic malignancies, even in early stage tumors. The higher recurrence rate after thymomectomy in stage II tumors, along with the difficulty in accurate clinical staging, indicate that thymectomy should still be recommended to ensure radical resection and accurate staging. And this is particularly true for thymoma patients with concomitant MG.
Acknowledgements
None.
Footnote
Members of Chinese Alliance for Research in Thymomas (ChART): Yi Shen, Yucheng Wei, Affiliated Hospital of Qingdao University, Qingdao, China; Yin Li, Guanghui Liang, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China; Keneng Chen, Hao Fu, Beijing Cancer Hospital, Beijing, China; Hezhong Chen, Shihua Yao, Changhai Hospital, Shanghai, China; Youbin Cui, Yanzhong Xin, First Affiliated Hospital of Jilin University, Changchun, China; Renquan Zhang, Ningning Kang, First Hospital of Anhui Medical University, Hefei, China; Lijie Tan, Jianyong Ding, Hao Wang, Gang Chen, Jie Wu, Zhongshan Hospital, Fudan University, Shanghai, China; Chun Chen, Wei Zheng, Fujian Medical University Union Hospital, Fuzhou, China; Liewen Pang, Fangrui Wang, Huashan Hospital, Fudan University, Shanghai, China; Yangchun Liu, Qing Lin, Jiangxi People’s Hospital, Nanchang, China; Yongyu Liu, Yongkai Wu, Liaoning Cancer Hospital, Shenyang, China; Wentao Fang, Jie Zhang, Yan Shen, Changlu Wang, Lei Zhu, Zhitao Gu, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Yongtao Han, Lin Peng, Sichuan Cancer Hospital, Chengdu, China; Jianhua Fu, Qianwen Liu, Department of Thoracic Surgery, Guangdong Esophageal Cancer Institute, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China; Zhentao Yu, Jie Yue, Tianjin Cancer Hospital, Tianjin, China; Peng Zhang, Yuan Chen, Tianjin Medical University General Hospital, Tianjin, China; Yun Wang, Yingcai Geng, West China Hospital Sichuan University, Chengdu, China; Xinming Zhou, Hongguang Zhao, Zhejiang Cancer Hospital, Hangzhou, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Onuki T, Ishikawa S, Iguchi K, et al. Limited thymectomy for stage I or II thymomas. Lung Cancer 2010;68:460-5. [Crossref] [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. [Crossref] [PubMed]
- Sakamaki Y, Kido T, Yasukawa M. Alternative choices of total and partial thymectomy in video-assisted resection of noninvasive thymomas. Surg Endosc 2008;22:1272-7. [Crossref] [PubMed]
- Nakajima J, Murakawa T, Fukami T, et al. Postthymectomy myasthenia gravis: relationship with thymoma and antiacetylcholine receptor antibody. Ann Thorac Surg 2008;86:941-5. [Crossref] [PubMed]
- Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000;55:16-23. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Marom EM, Rosado-de-Christenson ML, Bruzzi JF, et al. Standard report terms for chest computed tomography reports of anterior mediastinal masses suspicious for thymoma. J Thorac Oncol 2011;6:S1717-23. [Crossref] [PubMed]
- Marom EM, Milito MA, Moran CA, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol 2011;6:1274-81. [Crossref] [PubMed]
- Priola AM, Priola SM, Di Franco M, et al. Computed tomography and thymoma: distinctive findings in invasive and noninvasive thymoma and predictive features of recurrence. Radiol Med 2010;115:1-21. [Crossref] [PubMed]
- Benveniste MF, Moran CA, Mawlawi O, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol 2013;8:502-10. [Crossref] [PubMed]
- Roth T, Ackermann R, Stein R, et al. Thirteen years follow-up after radical transsternal thymectomy for myasthenia gravis. Do short-term results predict long-term outcome? Eur J Cardiothorac Surg 2002;21:664-70. [Crossref] [PubMed]
- Buckingham JM, Howard FM Jr, Bernatz PE, et al. The value of thymectomy in myasthenia gravis: a computer-assisted matched study. Ann Surg 1976;184:453-8. [Crossref] [PubMed]
- Ito M, Fujimura S, Monden Y, et al. A retrospective group study on post-thymectomy myasthenia gravis. Nihon Kyobu Geka Gakkai Zasshi 1992;40:189-93. [PubMed]
- Sonett JR, Jaretzki A 3rd. Thymectomy for nonthymomatous myasthenia gravis: a critical analysis. Ann N Y Acad Sci 2008;1132:315-28. [Crossref] [PubMed]