Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment
Overview of restenosis
Restenosis is defined as a reduction in lumen diameter after percutaneous coronary intervention (PCI), either with or without stent implantation. In case of no-stent strategy, it usually consists in vessel remodeling and elastic recoil (ER); otherwise it is determined by an excessive tissue proliferation in the luminal vessel of the stent called “neointimal proliferation”, or by a new-occurring atherosclerotic process called “neoatherosclerosis” (1).
From the clinical point of view, restenosis is often associated with the recurrence of angina symptoms or an acute coronary syndrome, and may drive to a reintervention either with coronary artery bypass or re-PCI. This reintervention is usually called target lesion revascularization (TLR) (2). However, we have to distinguish between those revascularizations that are performed for an incidental finding during angiography (angiographic-driven TLR) and those that are clinically-driven because of symptoms or evidence of a significant ischemia during provocative tests.
In-stent restenosis (ISR) has always been considered the “enemy” for the interventional cardiologists, thus many technical improvements in the last 20 years aimed at reducing its occurrence: firstly, newer generation bare metal stents (BMS) (3), then drug-eluting stents (DES) (4) and finally drug-coated balloons (DCB) (5,6).
Moreover, ISR is an independent predictor for mortality during follow-up, together with other relevant clinical factors as age, sex, diabetes mellitus, smoke habit, previous by-pass surgery, and left ventricular ejection fraction (7).
To establish the exact incidence of restenosis is not easy, depending on a number of different factors and variables. In the pre-stent era it ranged between 32–55% of all angioplasties, and drop to 17–41% (8-11) in the BMS era (12-14). A further step to reduce restenosis was undertaken with the advent of DES, with a reduction to numbers <10% (15,16). ISR rate appears to be higher when the patient has a multivessel disease rather than a single-vessel disease, as demonstrated in the study of Zhao: the occurrence of ISR was significantly higher in patients with two-vessel (OR: 2.922; 95% CI: 1.266–6.745; P=0.012) or three-vessel disease (OR: 2.574; 95% CI: 1.128–5.872; P=0.025) when compared with those with one-vessel disease (17).
Other than the drug eluted, newer generation DES were developed in order to further reduce restenosis and improve the overall performance thanks to improvements in the platform (e.g., thin-strut cobalt chromium vs. thick-strut stainless steel), polymer (thinner and/or biodegradable and/or its absence), and drug (biolimus A9 and zotarolimus were specifically designed for intracoronary use). Clinical data show the superiority of newer DES in terms of TLR, myocardial infarction and stent thrombosis (ST) (18,19).
All these improvements however have pushed the interventional cardiologist to treat patients that were previously reserved to surgical revascularization (i.e., left main stem, complex bifurcations and complex and extremely calcified lesions). As a consequence, real world registries, including more complex patients and lesions, show a higher rate of ISR if compared to the one that is shown by randomized trials.
Etiopathogenesis
Restenosis is a progressive phenomenon that begins in the early hours after the barotrauma determined by PCI (Table 1).
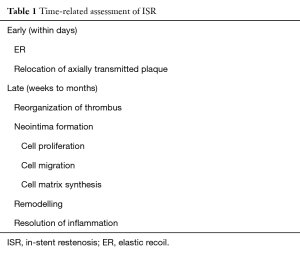
Full table
The three major pathogenic mechanisms that underlie restenosis are:
- Early elastic return (recoil);
- Vascular remodeling;
- Neointimal hyperplasia.
The first and the second mechanisms are typical of “old-style” angioplasty before the stent era. On the other hand, the presence of metallic struts promotes a new mechanism called neointimal hyperplasia.
Myointimal trauma induced by PCI affects the atherosclerotic process and changes its course from the natural evolution of atherosclerotic plaque to a more aggressive local response to the treatment. Atherosclerosis is characterized by a sequence of processes which induce vasoconstriction and the initial endothelial dysfunction resulting in the mechanism of ER and vascular remodelling, enhanced by an inflammatory process triggered by vessel injury, as evidenced by the increase in C-reactive protein or MCP-1 in patients at increased risk of restenosis (20).
ER and vascular remodeling
The internal and external elastic laminae (IEL/EEL) of human epicardial coronary arteries contain elastin fibres that may cause ER after balloon overstretch. ER can result in up to 40% loss of luminal areas and occurs between a few seconds up to minutes after balloon deflation.
Stenting reduces acute recoil thanks to scaffolding, despite both angioplasty and stent itself produce overstretch injury. Vascular remodeling is a complex phenomenon including also medial and/or adventitial response to injury. On the other hand, after balloon angioplasty the contribution of neointimal hyperplasia to restenosis is relatively limited, and lumen narrowing is mostly determined by vessel remodeling (21,22).
The first two mechanisms have been almost cancelled with the advent of stents compared to simple balloon angioplasty, but the presence of metallic struts favours the third mechanism represented by neointimal hyperplasia.
Neointimal hyperplasia
Vascular smooth muscle cell (VSMC) proliferation or migration and activation are caused by vessel injury following mechanical stretch, IEL rupture, and medial dissection. Also endothelial denudation and exposure to circulating mitogens (angiotensin II and plasmin) may have a role; moreover, platelets, endothelial cells, vessel smooth muscle and inflammatory cells may release mitogens and cytokines.
ISR is primarily a non-specific inflammatory response to vessel wall injury due to the persistent “insult” exercised by a foreign element as the metal struts of the stent. Chronic wall stress due to media damage and stent struts protrusion in tunica intima stimulates inflammatory processes and the migration of smooth muscle cells from tunica media and myofibroblasts from tunica adventitia to tunica intima. Simultaneously, the vessel discontinuity created by the stent struts may facilitate a contact between the two distal layers of the vessel wall with blood elements, resulting in the caption of various stimuli for neointimal proliferation. Confirmation of this hypothesis derives from the observation of a post-procedural increase of systemic markers of inflammation and the presence of inflammatory cells in the analyzed plaques (23,24).
On the background of this information, DES technology was developed, thanks to the addition of the following components to the metal structure of stents:
- An antimitotic/antiproliferative drug;
- A carrier/polymer.
The aim of these improvements was to counterbalance the excessive neointimal proliferation stimulated by the presence of metallic struts.
The polymer has the task of distributing the drug to the vessel wall and then remains intact on the struts surface; it was one of the major defendants for the increased rate of late and very late thrombotic events with first-generation DES. Therefore, newer generation DES was developed with polymers that were more biocompatible or biodegradable or even absent.
Currently, ISR predictors may be classified into three categories (Table 2): patient-related, lesion-related, procedural-related.

Full table
Patient-related factors
Diabetes mellitus has a major role in determining and foster the ISR process. The prothrombotic milieu typical of diabetic coronary vessels, including increased blood viscosity, decrease in biological activity of antithrombin II, fibrinogen and factor VIII and enhanced platelet aggregation, could play a role in this phenomenon (25,26). Furthermore, the effect of stimulatory growth factors like insulin-like growth factor-1 on VSMCs may cause a greater degree of neointimal hyperplasia (27). Atherectomy specimens from restenotic lesions in diabetic patients showed no increased proliferation of the smooth muscle, but rather a greater fibrotic response which may lead to vessel constriction (28). The process is more closely related to a genetic predisposition which may affect not only the mechanism of ISR but also the process of resistance to antirestenotic drugs issued by DES, conferring resistance to those drugs and their analogues (29,30). Zhao observed an interesting correlation between the ISR risk and insulin-resistance: patients with ISR had higher insulin resistance index than patients without (P=0.004) (17).
Variables like diabetes, hypertension, smoking and drug therapy may substantially change the association between patient genotype and disease increasing, decreasing or eliminating the genetic interactions observed in different populations: genetic polymorphism was evaluated in several studies, in particular CD18 gene polymorphism which reduces inflammatory cells adhesion MAC-1 related (31) or MMP3 gene promoter polymorphism, involving extracellular matrix degradation in the inflammatory process (32).
Lesion and procedural-related factors
Several factors determine an inhomogeneous drug distribution promoting the ISR process: vessel and lesion characteristics (a tortuous segment, a calcified vessel, a different caliber of the vessel segment or bifurcation lesions), possible stent struts fractures and finally stent underexpansion (33).
Lesion preparation in the DES era became of primary importance and correlated with short and long-term outcome. Among various lesion subsets, coronary calcification is one of the major determinants of PCI failure and has been correlated to late adverse events (34). Therefore, correct positioning of a DES is the crucial point for an optimal revascularization, avoiding adverse events which may occur in case of inadequate stent expansion: this complication may easily occur in case of incorrect preparation of the lesion, especially in case of complex or calcified ones.
Through the sole angiographic evaluation, it may be difficult to recognize an inadequate stent expansion, thus an invasive evaluation by intra-vascular-ultrasound (IVUS) or optical-coherence-tomography (OCT) allows a more detailed analysis of a correct implantation. For this purpose, the MUSIC criteria were developed: a stent expansion is defined excellent if minimum lumen area inside the stent is ≥90% of the average reference lumen area (35).
Stent malapposition can be:
- Acute, generally secondary to the technique of implantation and anatomical characteristics of coronary lesions such as calcified lesions, which do not allow an homogeneous and complete stent expansion, and those located in large coronary vessels;
- Acquired, whose pathogenesis includes:
- Positive remodeling of the vessel;
- Dissolution of the thrombus or partial regression of atherosclerotic plaque beneath the stent, which determine a continuous solution between stent and vessel wall;
- Chronic stent recoil.
However, stent malapposition is characterized by interposed blood between the stent struts and the vessel wall, and predisposes to ST rather than ISR (36).
A correct stent deployment may greatly influence the ISR risk: predilatation by a semi-compliant balloon should be carried out, especially in case of complex, type B2/C lesions; important, the balloon should be shorter than the DES, in order to treat all the diseased and predilated segment with the drug eluted. Post-dilation should be performed anytime the operator is not sure about correct stent expansion and possible areas of malapposition (15).
Stent length is an important determinant for ISR as well; in fact longer stents are an important risk factor for restenosis and ST. Choi in a study with median follow-up of 36.9 months observed how patients treated with stent length ≥32 mm had a greater risk of ISR than those treated with a stent <32 mm (37). Finally, also vessel diameter plays an important role: as reported by the HORIZONS-AMI study, ISR rate increases significantly when the vascular caliber is ≤3 mm (38).
Neoatherosclerosis
Atherosclerotic lesions may affect the nascent neointima in the meaning of a new disease known as neoatherosclerosis, responsible for a certain number of stent failures (including ISR, acute coronary syndromes and ST, both for BMS or DES) over the time (39,40). Little is known about the predisposition, pathogenesis and development of neoatherosclerosis.
One of the involved mechanisms depends on an incomplete regeneration of the endothelium, which causes an excessive uptake of circulating lipids from which derives an accelerated atherosclerosis of the nascent neointima (41,42). Neoatherosclerosis is less frequent in BMS as compared with DES, so that the drug released from the latter seems to be one of the causative factors due to the incomplete endothelialization related to the drug itself (43,44).
Nakazawa et al. have individuated some independent predictors for its occurrence: younger age, longer stent age (≥48 months), sirolimus-eluting stent or paclitaxel-eluting stent, active smoking, chronic kidney disease and angiotensin-converting enzyme inhibitors or angiotensin receptor blocker, or LDL-cholesterol levels above 70 mg/dL (43). According to the different definitions of neoatherosclerosis, its occurrence is difficult to be estimated. Taniwaki et al. reported an overall frequency, definite as a longitudinal extension of at least 1.0 mm in length using OCT analysis (excluding macrophage accumulation and fibrin deposition), of 40.9% at 5-year follow-up (45).
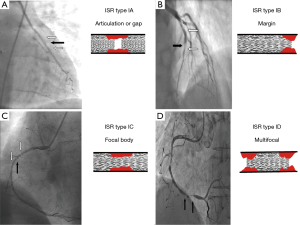
Classification of ISR
The most widely used classification for ISR is reported in Table 3 and Figures 1,2 (46). Goldberg describes a particular type of ISR identified as the “aggressive restenosis”, defined as: (I) an increase in lesion length; or (II) a decrease in minimal lumen diameter (MLD) at the time of ISR compared with baseline.
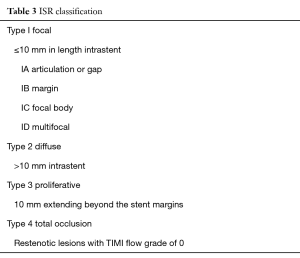
Full table
In a study performed to investigate the causes and patterns of ISR (diffuse or aggressive ISR), lesions with aggressive restenosis showed greater late lumen loss (LLL, defined as the difference between the MLD immediately after the procedure and the MLD at angiographic follow-up) (2.2±0.7 vs. 1.9±0.6, P<0.0001), despite lesser acute gain during the intervention (2.1±0.7 vs. 2.4±0.6, P<0.0001). Aggressive ISR occurred earlier and was more common in women, in shorter lesions and with larger baseline MLD (47).
How to treat ISR
The introduction of DES has drastically reduced the occurrence of severe neointimal proliferation, the dominant cause of ISR. This decrease translated into important reductions in TLR (48). Newer DES are considered safer than the first generation DES (49,50), however the ISR rate is still not negligible and the treatment of this complication is today an interesting challenge for the interventional cardiologist.
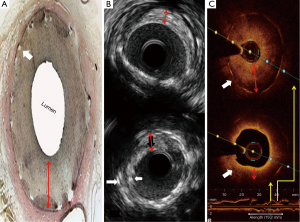
IVUS imaging allows a real-time assessment of lumen area and plaque composition, size, and distribution (Figure 3). Findings from meta-analyses suggested that better clinical and angiographic results may be obtained under IVUS guidance when treating patients with ISR (52). A recent retrospective and observational study suggested that OCT-guided stenting might improve clinical outcomes as well. Owing to its very high resolution (Figure 3), OCT is used to reveal the underlying mechanisms in patients with stent failure, including ISR and ST (53).
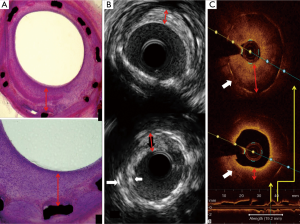
Regarding the optimal treatment strategy, the 2014 European guidelines (54) suggest to use another DES (class I, level of evidence A), considering improved results if compared to those obtained with balloon angioplasty, BMS implantation or brachytherapy (55,56). Treatment of DES-ISR is associated with poorer late outcomes than that obtained after treatment for BMS-ISR, so repeat stenting with DES rapidly became established as the treatment of choice for DES-ISR (57).
In the RIBS III (restenosis intra-stent: balloon angioplasty versus drug-eluting stent) trial, a prospective multicenter registry including 363 patients with DES-ISR, the use of a hetero-DES approach was recommended, and outcomes were compared between patients who were treated according to this recommendation versus those who were not. The use of second-generation DES resulted superior to first generation ones, and also IVUS-guidance was associated with better long-term results (58).
To this day, the real innovation for the treatment of ISR, as also underlined by current guidelines, is represented by the use of DCB, both for the treatment of DES and BMS ISR: here this technology gained a role similar to DES (class I, level of evidence A) (54).
Historically, ISR represented the first clinical application for DCB. From the pathophysiological point of view, currently available DCB elute paclitaxel, a drug that effectively inhibit smooth muscle cells proliferation and migration by irreversibly stabilizing intracellular microtubules, thus blocking cell replication during the metaphase and anaphase stages of mitosis. The advantages of this drug include a high lipophilia, a relative selectivity for smooth muscle cells and cytotoxic action limited for a few days only (59).
The main advantage of DCB in the treatment of ISR is that no new stent scaffold is needed within the previously implanted stent (5). In 2006, the first human trial involving a DCB demonstrated the superiority of such an investigational device over plain balloon angioplasty in terms of in-segment LLL for the treatment of BMS restenosis. The superiority of DCB in terms of target vessel revascularization (TVR) persisted after 5 years (38.9% vs. 9.3%, P<0.004) (60).
After this first landmark study, other came and showed the value of this technique. Among the others, the intracoronary stenting and angiographic results: drug eluting stents for in-stent restenosis 3 (Treatment Approaches ISAR-DESIRE 3) trial demonstrated the noninferiority of DCB compared to paclitaxel-eluting stents in terms of percent diameter stenosis at 6–8 months follow-up, for the treatment of DES restenosis (61).
Alfonso et al. in a prospective, multicenter, randomized trial (restenosis intra-stent of bare metal stents: paclitaxel-eluting balloon vs. everolimus-eluting stent: RIBS V) compared DCB with EES in 189 patients with BMS-ISR (62). Primary endpoint was MLD at 9 months follow-up. Patients in the EES arm had a significantly larger MLD (2.36±0.6 vs. 2.01±0.6 mm; 95% CI: 0.16–0.53, P<0.001) but LLL was similar in the two study groups (0.04±0.5 mm in DES vs. 0.14±0.5 mm in DCB group, P=0.14). Binary restenosis (4.7% vs. 9.5%, P=0.22) was very low and similar in both groups, like the occurrence of the combined clinical outcome measure (cardiac death, myocardial infarction, and TVR): 6% vs. 8%; HR: 0.76; 95% CI: 0.26–2.18, P=0.6).
Another interesting study by Alfonso and his group is RIBS IV (63) in which 309 patients overall with DES ISR were randomly assigned a 2nd generation DES, namely everolimus-eluting stent (Xience Prime, Abbott Vascular; US, n=155) or DCB (SeQuent Please, B. Braun; Germany, n=154). EES provided better primary endpoint consisting in in-segment MLD at the 6- to 9-month angiographic follow-up. In fact, patients in the EES arm had a significantly larger MLD (2.03±0.7 vs. 1.80±0.6 mm; P<0.01) (absolute mean difference: 0.23 mm; 95% CI: 0.07–0.38), net lumen gain (1.28±0.7 vs. 1.01±0.7 mm; P<0.01), and lower percent diameter stenosis (23%±22% vs. 30%±22%; P<0.01) and binary restenosis rate (11% vs. 19%; P=0.06), compared with patients in the DEB arm. At the 1-year clinical follow-up (100% of patients), the main clinical outcome measure (composite of cardiac death, myocardial infarction, and TVR) was significantly reduced in the EES arm (10% vs. 18%; P=0.04; hazard ratio: 0.58; 95% CI: 0.35–0.98), mainly driven by a lower need for TVR (8% vs. 16%; P=0.035).
In this light, authors concluded that in patients with DES-ISR, EES provided superior long-term clinical and angiographic results compared with DEB because both outcomes showed better results for EES compared with DCB but the study have some bias. If truth be told that 2nd generation DES results better than 1st generation DCB. We well known that DCB are different each other and there is not demonstrable a class effect. So we think that this sentence is not conclusive. Again, RIBS IV cohort is still too small to give a conclusive result (309 patients overall), newer generation DCB should be tested in this challenging setting, furthermore, DCB presents the great advantage to avoid the onion-skin phenomenon in which new metal layer is applied to previous implanted DES. Alfonso commented his study: “Treatment of DES in-stent restenosis remains challenging and associated with poorer clinical and angiographic results than treatment of bare-metal stent in-stent restenosis, further studies including more patients and longer follow-up are still warranted in this adverse setting”.
Recently, Siontis et al. published a meta-analysis (64) on different strategies for the treatment of ISR, including 5,923 patients from 27 trials with a follow-up ranging from 6 to 60 months. The primary endpoint was percent diameter stenosis at angiographic follow-up. Based on the results of this meta-analysis, everolimus-eluting stent resulted the most effective treatment of ISR according to clinical and angiographic outcomes with a difference of −9.0% diameter stenosis (95% CI: −15.8 to −2.2) vs. DCB, −9.4% vs. sirolimus-eluting stents, −10.2% vs. paclitaxel-eluting stents, −19.2% vs. brachytherapy, −23.4% vs. BMSs, −24.2% vs. balloon angioplasty, and −31.8% (range, −44.8% to −18.6%) vs. rotablation. DCB resulted the second most effective treatment, and authors concluded that “two strategies should be considered for treatment of any type of coronary ISR: PCI with everolimus-eluting stents because of the best angiographic and clinical outcomes, and DCB because of its ability to provide favourable results without adding a new stent layer” (64).
Table 4 shows a summary of the most relevant studies that investigated DCB for BMS/DES restenosis.
Full table
Key points and conclusions
An analysis of available data from the literature brings to the following the key points:
- Restenosis represents still “the present” and not “the past” of interventional cardiology. Specifically, ISR is the most diffuse and important form of restenosis, a still open challenge also in the DES era;
- Regardless of the stent type, small vessel size and stent length are the most important predictors of ISR;
- Restenosis is an independent predictor for mortality during follow-up together with other relevant clinical factors like age, sex, diabetes mellitus, smoke habit, previous by-pass surgery, and left ventricular ejection fraction;
- Just like DES, DCB is currently the first choice treatment for this complication, both in case of BMS or DES restenosis. Despite a similar clinical outcome, DCB spares the need for adding further metal struts on a pre-existing stent, whose chronic inflammatory reaction and its consequences are still unknown.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Eeckhout E, Serruys PW, Wijns W, et al. editors. Percutaneous Interventional Cardiovascular Medicine: the PCR - EAPCI Textbook. Europa Ed. PCR Publ., 2012:785-826.
- Alfonso F, Byrne RA, Rivero F, et al. Current treatment of in-stent restenosis. J Am Coll Cardiol 2014;63:2659-73. [Crossref] [PubMed]
- Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994;331:496-501. [Crossref] [PubMed]
- Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 2009;119:3198-206. [Crossref] [PubMed]
- Cortese B, Berti S, Biondi-Zoccai G, et al. Drug-coated balloon treatment of coronary artery disease: a position paper of the Italian Society of Interventional Cardiology. Catheter Cardiovasc Interv 2014;83:427-35. [Crossref] [PubMed]
- Cortese B, Bertoletti A. Paclitaxel coated balloons for coronary artery interventions: a comprehensive review of preclinical and clinical data. Int J Cardiol 2012;161:4-12. [Crossref] [PubMed]
- Cassese S, Byrne RA, Schulz S, et al. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J 2015;36:94-9. [Crossref] [PubMed]
- Fishman RF, Kuntz RE, Carrozza JP Jr, et al. Long-term results of directional coronary atherectomy: predictors of restenosis. J Am Coll Cardiol 1992;20:1101-10. [Crossref] [PubMed]
- Le Feuvre C, Bonan R, Lespérance J, et al. Predictive factors of restenosis after multivessel percutaneous transluminal coronary angioplasty. Am J Cardiol 1994;73:840-4. [Crossref] [PubMed]
- Bakhai A, Booth J, Delahunty N, et al. The SV stent study: a prospective, multicentre, angiographic evaluation of the BiodivYsio phosphorylcholine coated small vessel stent in small coronary vessels. Int J Cardiol 2005;102:95-102. [Crossref] [PubMed]
- Agostoni P, Valgimigli M, Biondi-Zoccai GG, et al. Clinical effectiveness of bare-metal stenting compared with balloon angioplasty in total coronary occlusions: insights from a systematic overview of randomized trials in light of the drug-eluting stent era. Am Heart J 2006;151:682-9. [Crossref] [PubMed]
- Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 2007;370:937-48. [Crossref] [PubMed]
- Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 2007;356:1030-9. [Crossref] [PubMed]
- García Del Blanco B, Rumoroso Cuevas JR, Hernández Hernández F, et al. Spanish cardiac catheterization and coronary intervention registry. 22nd official report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2012). Rev Esp Cardiol (Engl Ed) 2013;66:894-904. [Crossref] [PubMed]
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23. [PubMed]
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221-31. [Crossref] [PubMed]
- Zhao LP, Xu WT, Wang L, et al. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron Artery Dis 2015;26:5-10. [Crossref] [PubMed]
- Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 2010;375:201-9. [Crossref] [PubMed]
- Harjai KJ, Kondareddy S, Pinkosky B, et al. Everolimus-eluting stents versus sirolimus- or paclitaxel-eluting stents: two-year results from the Guthrie Health Off-Label Stent (GHOST) registry. J Interv Cardiol 2013;26:153-62. [Crossref] [PubMed]
- Angioi M, Abdelmouttaleb I, Rodriguez RM, et al. Increased C-reactive protein levels in patients with in-stent restenosis and its implications. Am J Cardiol 2001;87:1189-93; A4.
- Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation 1994;89:2816-21. [Crossref] [PubMed]
- Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation 1996;94:35-43. [Crossref] [PubMed]
- Piek JJ, van der Wal AC, Meuwissen M, et al. Plaque inflammation in restenotic coronary lesions of patients with stable or unstable angina. J Am Coll Cardiol 2000;35:963-7. [Crossref] [PubMed]
- Farb A, Weber DK, Kolodgie FD, et al. Morphological predictors of restenosis after coronary stenting in humans. Circulation 2002;105:2974-80. [Crossref] [PubMed]
- MacRury SM, Lowe GD. Blood rheology in diabetes mellitus. Diabet Med 1990;7:285-91. [Crossref] [PubMed]
- Ostermann H, van de Loo J. Factors of the hemostatic system in diabetic patients. A survey of controlled studies. Haemostasis 1986;16:386-416. [PubMed]
- Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol 1996;27:528-35. [Crossref] [PubMed]
- Murcia AM, Fallon JT, Fuster V. Smooth muscle cell proliferation does not account for restenosis in diabetic patients. Circulation 1196:I619-20.
- Yusuf RZ, Duan Z, Lamendola DE, et al. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets 2003;3:1-19. [Crossref] [PubMed]
- Huang S, Houghton PJ. Mechanisms of resistance to rapamycins. Drug Resist Updat 2001;4:378-91. [Crossref] [PubMed]
- Koch W, Böttiger C, Mehilli J, et al. Association of a CD18 gene polymorphism with a reduced risk of restenosis after coronary stenting. Am J Cardiol 2001;88:1120-4. [Crossref] [PubMed]
- Humphries S, Bauters C, Meirhaeghe A, et al. The 5A6A polymorphism in the promoter of the stromelysin-1 (MMP3) gene as a risk factor for restenosis. Eur Heart J 2002;23:721-5. [Crossref] [PubMed]
- Fujii K, Mintz GS, Kobayashi Y, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation 2004;109:1085-8. [Crossref] [PubMed]
- Costa JR Jr, Sousa A, Moreira AC, et al. Incidence and predictors of very late (>or=4 years) major cardiac adverse events in the DESIRE (Drug-Eluting Stents in the Real World)-Late registry. JACC Cardiovasc Interv 2010;3:12-8. [Crossref] [PubMed]
- de Jaegere P, Mudra H, Figulla H, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study). Eur Heart J 1998;19:1214-23. [Crossref] [PubMed]
- Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 2007;115:2426-34. [Crossref] [PubMed]
- Choi IJ, Koh YS, Lim S, et al. Impact of the stent length on long-term clinical outcomes following newer-generation drug-eluting stent implantation. Am J Cardiol 2014;113:457-64. [Crossref] [PubMed]
- Stone GW, Parise H, Witzenbichler B, et al. Selection criteria for drug-eluting versus bare-metal stents and the impact of routine angiographic follow-up: 2-year insights from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol 2010;56:1597-604. [Crossref] [PubMed]
- Taniwaki M, Windecker S, Räber L. Neoatherosclerosis as reason for stent failures beyond 5 years after drug-eluting stent implantation. Eur Heart J 2014;35:1980. [Crossref] [PubMed]
- Lee SY, Hur SH, Lee SG, et al. Optical coherence tomographic observation of in-stent neoatherosclerosis in lesions with more than 50% neointimal area stenosis after second-generation drug-eluting stent implantation. Circ Cardiovasc Interv 2015;8:e001878. [Crossref] [PubMed]
- Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol 2008;52:333-42. [Crossref] [PubMed]
- Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014;129:211-23. [Crossref] [PubMed]
- Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 2011;57:1314-22. [Crossref] [PubMed]
- Yonetsu T, Kato K, Kim SJ, et al. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging 2012;5:660-6. [Crossref] [PubMed]
- Taniwaki M, Windecker S, Zaugg S, et al. The association between in-stent neoatherosclerosis and native coronary artery disease progression: a long-term angiographic and optical coherence tomography cohort study. Eur Heart J 2015;36:2167-76. [Crossref] [PubMed]
- Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation 1999;100:1872-8. [Crossref] [PubMed]
- Goldberg SL, Loussararian A, De Gregorio J, et al. Predictors of diffuse and aggressive intra-stent restenosis. J Am Coll Cardiol 2001;37:1019-25. [Crossref] [PubMed]
- Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med 2013;368:254-65. [Crossref] [PubMed]
- Park KW, Kang SH, Velders MA, et al. Safety and efficacy of everolimus- versus sirolimus-eluting stents: a systematic review and meta-analysis of 11 randomized trials. Am Heart J 2013;165:241-50.e4. [Crossref] [PubMed]
- Minha S, Barbash IM, Dvir D, et al. Second-generation everolimus-eluting stents compared to first-generation drug-eluting stents in patients treated for multivessel disease. J Interv Cardiol 2013;26:561-9. [Crossref] [PubMed]
- Takimura CK, Campos CA, Melo PH, et al. Preclinical study of a biodegradable polymer-based stent with abluminal sirolimus release. Arq Bras Cardiol 2014;102:432-40. [PubMed]
- Zhang Y, Farooq V, Garcia-Garcia HM, et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention 2012;8:855-65. [Crossref] [PubMed]
- Alfonso F, Dutary J, Paulo M, et al. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart 2012;98:1213-20. [Crossref] [PubMed]
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Kastrati A, Mehilli J, von Beckerath N, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA 2005;293:165-71. [Crossref] [PubMed]
- Mehilli J, Byrne RA, Tiroch K, et al. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J Am Coll Cardiol 2010;55:2710-6. [Crossref] [PubMed]
- Byrne RA, Cassese S, Windisch T, et al. Differential relative efficacy between drug-eluting stents in patients with bare metal and drug-eluting stent restenosis; evidence in support of drug resistance: insights from the ISAR-DESIRE and ISAR-DESIRE 2 trials. EuroIntervention 2013;9:797-802. [Crossref] [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Dutary J, et al. Implantation of a drug-eluting stent with a different drug (switch strategy) in patients with drug-eluting stent restenosis. Results from a prospective multicenter study (RIBS III [Restenosis Intra-Stent: Balloon Angioplasty Versus Drug-Eluting Stent]). JACC Cardiovasc Interv 2012;5:728-37. [Crossref] [PubMed]
- Gray WA, Granada JF. Drug-coated balloons for the prevention of vascular restenosis. Circulation 2010;121:2672-80. [Crossref] [PubMed]
- Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med 2006;355:2113-24. [Crossref] [PubMed]
- Byrne RA, Neumann FJ, Mehilli J, et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet 2013;381:461-7. [Crossref] [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent). J Am Coll Cardiol 2014;63:1378-86. [Crossref] [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol 2015;66:23-33. [Crossref] [PubMed]
- Siontis GC, Stefanini GG, Mavridis D, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet 2015;386:655-64. [Crossref] [PubMed]


