Influence of old pulmonary tuberculosis on the management of secondary spontaneous pneumothorax in patients over the age of 70 years
Introduction
Secondary spontaneous pneumothorax (SSP) usually develops in elderly patients with underlying lung disease. Owing to pulmonary and general comorbidities, the management of elderly patients with SSP has not yet been standardized, nor has it resulted in satisfactory outcomes. Gupta et al. reported a striking increase of mortality from pneumothorax among patients aged over 55 years (1).
Among various predisposing lung pathologies of SSP, pulmonary tuberculosis (TB) is known as a major contributing factor after chronic obstructive pulmonary disease (COPD). In addition, pulmonary TB remains a significant public health burden in developing countries. In 2014, 9.6 million (133/105) new patients were diagnosed with TB worldwide. Unfortunately, South Korea is still one of the high prevalence countries, with 86 per 100,000 patients (2). According to a report, the presence of old pulmonary TB is considered the main obstacle to the management of patients with SSP (3).
However, few reports have been published on the clinical impacts of SSP associated with old pulmonary TB in elderly patients. In this study, we aimed to investigate the outcomes of elderly patients (over 70 years) with SSP and evaluate the influence of old pulmonary TB on the management of those patients.
Methods
Study population and clinical data
We conducted a retrospective analysis of patients treated for SSP in our hospital between January 2002 and December 2014. This study was approved by the regional Institutional Review Board. The exclusion criteria were as follows: (I) patients under the age of 70 years at the time of their first occurrence of pneumothorax; patients with (II) traumatic pneumothorax; (III) iatrogenic pneumothorax; (IV) pneumothorax with concurrent lung malignancies; or (V) pneumothorax accompanying acute pulmonary infections; and (VI) bedridden patients.
We collected clinical data regarding age, sex, body weight, height, body mass index (BMI), Eastern Cooperative Oncology Group (ECOG) performance status, smoking status, previous obtained value of pulmonary function test, laboratory test results (sodium, protein, and albumin), and underlying comorbidities including diabetes, hypertension, cerebral infarction, cardiovascular disease, and COPD. On the basis of smoking history, patients were categorized as current smokers and non-smokers (never or ex-smoker). Smoking amount was described as the number of cigarette pack-years. The pulmonary function test was not routinely performed because the obtained value did not reflect the patients’ pulmonary function precisely due to air leakage. History of pulmonary TB was completely based on the medical records and chest computed tomography (CT). We think this is reliable because our country still has a relatively high prevalence of TB, which mandates routine check-up for its presence. The CT images were also evaluated for features of pulmonary lesions such as pleural adhesion, pleural thickening, scattered calcified nodules, parenchymal fibrotic bands, cavities, and scars that were regarded as old pulmonary TB (4,5).
The modified Goddard visual scoring system proposed by Hunsaker et al. in which the lung was divided into four zones—an upper and a lower zone on each side of the carina—was used in the present study (6,7). The scores represented the percentage of emphysematous destruction in each zone: 1 for 0–25%, 2 for 26–50%, 3 for 51–75%, and 4 for 76–100%. The final score was obtained by adding the individual scores of each of the four zones, with four being the minimum visual score and 16 being the maximum visual score. The CT images were reviewed and scored independently by two qualified thoracic surgeons who were blinded to any clinical data. After the independent evaluations, the two reviewers arrived at the final Goddard score that was agreed upon by discussion.
Treatment strategies
In our institution, the management strategy for patients with SSP was based on the American College of Chest Physicians (ACCP) and British Thoracic Society guidelines (8-10). Every patient had a chest tube inserted using 7F-30F thoracic catheters at the time of diagnosis of pneumothorax. If the drainage system was inadequate, the chest tube revision, reposition, change to larger caliber catheter, or second tube insertion was carried out. Suction to a chest tube was not routinely employed, but sometimes applied at −10 to −20 cmH2O for the failure of re-expansion or persistent air leak. The chest tubes were removed if the lung was fully expanded and there was no air leakage for at least a day. In some cases in which the lung remained expanded but air leakage persisted, the patient was discharged with a Heimlich valve for chest drainage. Although the surgical timing and method were at the discretion of the attending surgeons, we considered the general surgical indications to be the following: ipsilateral or contralateral recurrent pneumothorax, persistent air leakage (>7 days), poor expansion of the ipsilateral lung with massive air leakage, and suitable performance status for surgery. During the surgery, the air leak sources were routinely detected by lung inflation followed by underwater submersion. The basic principle of the surgery was to excise the target bullae, which were thin walled, white, translucent, or responsible for air leakage. We did not perform any further resection or repair of thick-walled, unruptured bullae regardless of the severity and distribution of the bullous emphysema. Adhesiolysis was also minimized to reduce any unexpected air leakage resulting from a visceral pleural injury. Bullectomy was mostly performed using stapling devices. Manual bullectomy and suture closure were performed via minithoracotomy or thoracotomy when the stapling device was not available for multifocal or dense pleural adhesion. Endoloop ligation was sometimes applied when the ruptured bulla was huge with a narrow stalk. The resection margin was covered with absorbable cellulose mesh and fibrin glue. Chemical or mechanical pleurodesis was not routinely conducted to reduce postoperative pain and complications in the elderly patients.
Follow-up
Patients were routinely followed-up for 1–2 weeks after discharge. The majority of patients were then referred back to other departments in our hospital for their underlying comorbidities. Long-term data were obtained from the latest available information via recent radiologic findings, medical records, or telephone interviews. Survival and the date of death were confirmed by using data from the National Health Insurance Service of our country. However, the cause of death could not be identified because almost all of the patients’ families were reluctant to disclose this information.
Statistical analyses
Statistical evaluation was performed using the Statistical Package for the Social Sciences program version 22.0 for Windows (SPSS, Chicago, IL, USA). Discrete variables were analyzed using Pearson’s chi-square test. Continuous variables were analyzed using Student’s t-test. Recurrence and survival analyses of the 133 patients—81 without old pulmonary TB and 52 with old pulmonary TB—was performed using the Kaplan-Meier method. Recurrence-free survival rate was calculated from the date of first occurrence of SSP to the date of first recurrence or the date of last follow-up. Overall survival rate was calculated from the date of first occurrence of SSP to the date of any cause of death or the date of last follow-up. The differences in survival rates between subgroups were assessed using the log-rank test. A statistically significant difference was defined as a two-sided P value of less than 0.05.
Results
During the study period, a total of 216 consecutive SSP cases recruited under the inclusion and exclusion criteria for the present study. We classified the study population into two groups according to the presence of old pulmonary tuberculosis [non-tuberculosis (NTB) group vs. TB group]. Of these, 134 (62.0%) did not have old pulmonary TB and the other 82 (38.0%) had experienced pulmonary TB. Figure 1 shows treatment flow diagram of both groups in the study population. The mean follow-up period was 42.8±36.1 months, and 205 cases (94.9%) were in male patients. The demographic and clinical characteristics of all 216 cases in the NTB and TB groups are listed and compared in Table 1. There was no female case in TB group. Compared to cases in NTB group, cases in TB group were in statistically significantly younger patients who had more height, lesser hypertension and higher Goddard score.

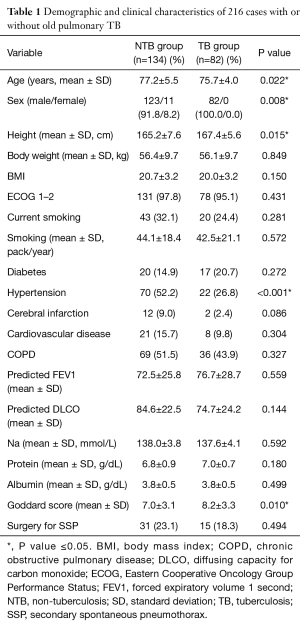
Full table
Table 2 shows various treatment outcomes associated with no surgical treatment and surgical treatment in both groups. In no surgical cases, the larger caliber of chest tubes were more frequently inserted for resolving pneumothorax in the TB group than the NTB group (P=0.027). Revision or repositioning of the chest tube was more frequently performed in the TB group, but there was no statistical significance. Among 46 cases that required surgery, 14 were non-video-assisted thoracoscopic surgery (VATS) cases including VATS with thoracotomy conversion, minithoracotomy, and conventional thoracotomy (Figure 1). Non-VATS was performed in 19.4% of cases in the NTB group and 53.3% of cases in the TB group (P=0.038). Preoperative and postoperative chest tube durations (CTD) were significantly longer in the TB group (P=0.024 and P=0.030, respectively) than in the NTB group. Mean time of surgery was also longer in the TB group than in the NTB group, but the difference was not statistically significant. Postoperative complications developed in 7 cases (46.7%) in the TB group, including four cases of postoperative prolonged air leak (>7 days), one of pneumonia, one of mechanical ventilator support for respiratory failure, and one of acute kidney injury. In the case of acute kidney injury, the patient died on postoperative day 6. In the NTB group, 5 (16.1%) cases developed postoperative complications, including three cases of postoperative prolonged air leak (>7 days), one of respiratory failure, and one of delirium. In seven patients with a postoperative prolonged air leak, chest tubes could be removed after few days without any further complications; three patients were discharged with Heimlich valve tubes. We could remove the three Heimlich tubes in an outpatient clinic in 1 or 2 weeks after the discharge. During the follow-up period, one patient in the TB group had an ipsilateral recurrence 7 years after the surgery; the recurrent pneumothorax was treated using closed tube thoracostomy.
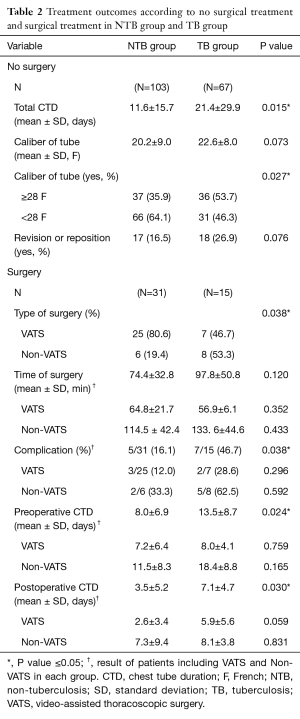
Full table
In our study, 133 patients (52 with old pulmonary TB and 81 without old pulmonary TB) had experienced 216 episodes of pneumothorax. Seventy-seven (57.9%) patients had a single episode of SSP, and 56 patients (42.1%) had recurrence of SSP during the follow-up period—37 patients with two episodes, 14 with three, 2 with four, and 3 with five. Seventeen patients (32.7%) with old pulmonary TB and 39 patients (48.1%) without old pulmonary TB experienced recurrences (P=0.105). One-year recurrence free survival rates were 72.5% and 60.8% for patients with and without old pulmonary TB, respectively (log rank, P=0.087) (Figure 2). Fifty-eight patients (26 with old pulmonary TB and 32 without old pulmonary TB) died during the follow-up period. In the total study population, overall 5-year survival rates were 59.7% and 61.8% for patients with and without old pulmonary TB, respectively (log rank, P=0.517) (Figure 3).
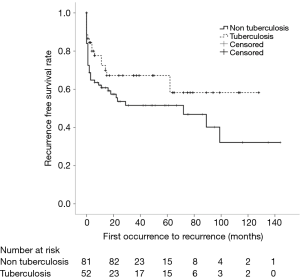
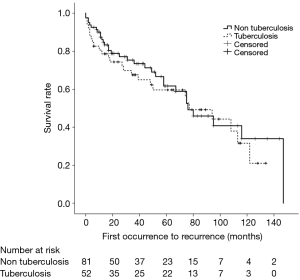
Discussion
Life expectancy of the elderly has been gradually improving for many decades (11), and in recent years, the elderly have been more commonly defined as individuals aged “70 years or more (12)”. Arias et al. reported that since 2009, the overall life expectancy at birth has been 78.5 years in the United States (13). As the elderly population keeps growing, the prevalence of COPD is also increasing worldwide. COPD has also been projected to increase in incidence to become the third leading cause of death by 2020 (14,15). Although tobacco smoking is widely accepted as a central factor in the development of COPD, treated pulmonary TB is also considered one of the most important predisposing factors in developing COPD (16).
TB remains a significant global health problem in spite of advancements in modern medical practices. In 2014, 9.6 million (133/105) new patients were diagnosed with TB and 1.5 million deaths occurred worldwide. The incidence of TB is reported to be 28/105 and 37/105 in the Americas and Europe region, respectively. However, unfortunately, Africa region and East-Asia region are still high prevalence areas with 281/105 and 211/105 patients, respectively (2).
SSP is associated with underlying pulmonary diseases, and is most frequently a consequence of COPD, especially the emphysema-dominant type of COPD. Most patients with SSP and concomitant COPD are older than 50 years, and patients who have more severe COPD tend to develop SSP more frequently (17). SSP is a potentially life-threatening disease because of the patient’s poor cardiopulmonary function and its association with various comorbidities.
Our study population did not include patients with SSP under the age of 70 years (based on the current definition of the elderly) (12), and about 40% of the study population had an experience of old pulmonary TB. In this study, mean Goddard score was statistically higher in the TB group than in the NTB group, although cases of the TB group were in more young patients. This result might support that the treated pulmonary TB is one of the important predisposing factors in developing COPD.
Another important purpose of this study was to investigate the influence of old pulmonary TB on the treatment of SSP. In patients without surgical treatment, the mean total CTD in the TB group was about two times longer than that in the NTB group. In patients with surgical treatment, non-VATSs were performed more frequently, and the total time of surgery and postoperative CTD were longer in the TB group. These results could be attributed to the fact that old pulmonary TB sequelae, such as pleural adhesion, pulmonary calcified nodules, or pulmonary fibrosis, sudden bronchopleural fistula and empyema, might be critical factors that make the treatment difficult and prolong the healing of lung parenchyma in both non-surgical and surgical treatment in the TB group. Interestingly, preoperative CTD was also more prolonged in the TB group than in the NTB group. We presume that the reasons were as follows: (I) surgeons took a long time to determine surgery for SSP in patients with old pulmonary TB sequelae that might result in unsatisfactory postoperative courses; and (II) patients and their families hesitated and needed more time to agree to surgery because of fear of poor surgical results caused by old pulmonary TB.
The morbidity and mortality rates were reported to be 19–38% and 0–9%, respectively, in some reports on patients with SSP who underwent surgical treatment. The postoperative recurrence rates were reported to range from 0% to 16% (18-22). In this study, we performed surgeries in carefully selected 46 (21.3%) out of the total 216 consecutive cases. The presence of old pulmonary TB was not a significant factor in determining the necessity for surgery. The incidence of postoperative morbidity was 16.1% (5/31 patients) in the NTB group and 46.7% (7/15 patients) in the TB group. The total incidence of postoperative mortality was 2.2% (1 patient in the TB group) and the postoperative recurrence rate was 2.2% (1 patient in the TB group). No patients underwent repeat surgeries for any complications or SSP recurrence. Recurrence-free survival rate and overall survival rate did not differ between the TB and NTB groups. We may consider our postoperative results to be favorable and comparable to those from previous reports, but the results were generally worse in patients from the TB group.
Although surgery is widely accepted as the most effective treatment for SSP, many thoracic surgeons refrain from performing surgery on elderly patients because of their short natural life expectancy and potential postoperative morbidity and mortality. However, the health status and general performance of the elderly population is now extremely varied—there are marathon runners older than 80 years as well as bed-ridden patients. Therefore, in recent years, age alone has not been used as a criterion for any type of surgery for SSP. Several studies have shown favorable outcomes by applying the surgical principle of minimal repair in elderly patients with SSP (20,21,23,24). Surgeries following this principle aim to excise only the targeted, active leakage point, and avoid performing a bullectomy of thick-walled bullae or a lung-volume-reduction surgery. The minimal repair principle can be well applied when there is no pleural adhesion at the active air leakage point. However, it might be hard for surgeons to resect target lesions with diffuse or dense pleural adhesion around the active air leakage point during surgery, especially in patients with pulmonary or pleural sequelae. Therefore, surgery should be given additional meticulous attention to have favorable postoperative courses in elderly SSP patients with old pulmonary TB.
Surgery is an effective treatment option in elderly patients with SSP, but unfortunately, the majority of elderly patients with SSP were considered inoperable. Therefore, conservative treatments have to be regarded as an important topic in elderly patients with SSP. Sahn et al. reported that the recurrence rate for SSP was between 39% and 47% in their review article (25). Several risk factors for recurrence of SSP have been reported including pulmonary fibrosis, age greater than 60 years, and patients without pleurodesis (10,25,26). Pleurodesis or a Heimlich valve may be appropriate for inoperable SSP (10). Our study showed a somewhat high percentage (about 40%) of recurrence rate in patients without surgery. All patients were over 70 years old in this study population. Pleurodesis was not performed in most patients with or without surgery, while Heimlich valve tube was mostly applied for patients with persistent air leakage because of concern for complications of pleurodesis, such as pain, fever, and respiratory failure, in very elderly patients. It might be a major weak point of this study that the analysis of treatment efficacy of pleurodesis could not be conducted in elderly patients with SSP. There is still a lack of definitive recommendation regarding pleurodesis in very elderly patients with SSP, although pleurodesis has been widely recommended to prevent recurrence of SSP and reduce the duration of air leakage (8,10,27). Therefore, a larger number of cases of elderly patients with well-designed prospective, randomized, controlled trials should be investigated in the future.
Study limitations
This study has several limitations. First, this study was a retrospective analysis. Second, the statistical power may be weak because the number of subgroups was small. Third, the presence of old pulmonary TB was dependent on the history description obtained from patients or family members in our medical records. It was impossible to identify the results of serologic or bacteriologic tests conducted and subscription data obtained decades ago. However, we believe that the public awareness of TB is high enough so that the patients know their own medical history about TB, especially because the nationwide TB survey has been conducted every 5 years since the 1960s in South Korea. Fourth, in addition to COPD and TB, many diffuse lung diseases, such as interstitial pneumonia, can be involved in SSP. Unfortunately, further analyses were not performed, related to the different pulmonary diseases in this study.
Conclusions
Among elderly patients with SSP, those in the TB group had a significantly longer CTD and significantly more postoperative complications than did patients in the NTB group. Moreover, thoracoscopic surgery could be performed more commonly in the NTB group than in the TB group. However, recurrence-free survival rate and overall survival rate after the first occurrence of SSP did not differ between the TB and NTB groups. We consider surgery could be conducted with acceptable morbidity and mortality in highly selected patients regardless of the presence of old pulmonary TB, even though such patients might have a more complicated clinical course.
Acknowledgements
The authors would like to thank Hee Jung Kim and Dae Sung Kim for data collection.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the regional Institutional Review Board (KNUH 2015-05-025) and written informed consent was obtained from all patients.
References
- Gupta D, Hansell A, Nichols T, Duong T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [Crossref] [PubMed]
- Global tuberculosis report 2015: World Health Organization, 2015. Available online: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf
- Freixinet JL, Caminero JA, Marchena J, et al. Spontaneous pneumothorax and tuberculosis: long-term follow-up. Eur Respir J 2011;38:126-31. [Crossref] [PubMed]
- Lee KS, Im JG. CT in adults with tuberculosis of the chest: characteristic findings and role in management. AJR Am J Roentgenol 1995;164:1361-7. [Crossref] [PubMed]
- Roy M, Ellis S. Radiological diagnosis and follow-up of pulmonary tuberculosis. Postgrad Med J 2010;86:663-74. [Crossref] [PubMed]
- Goddard PR, Nicholson EM, Laszlo G, et al. Computed tomography in pulmonary emphysema. Clin Radiol 1982;33:379-87. [Crossref] [PubMed]
- Hunsaker AR, Ingenito EP, Reilly JJ, et al. Lung volume reduction surgery for emphysema: correlation of CT and V/Q imaging with physiologic mechanisms of improvement in lung function. Radiology 2002;222:491-8. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. [Crossref] [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [Crossref] [PubMed]
- Lubitz J, Cai L, Kramarow E, et al. Health, life expectancy, and health care spending among the elderly. N Engl J Med 2003;349:1048-55. [Crossref] [PubMed]
- Bravo-Iñiguez C, Perez Martinez M, Armstrong KW, et al. Surgical resection of lung cancer in the elderly. Thorac Surg Clin 2014;24:371-81. [Crossref] [PubMed]
- Arias E. United States life tables, 2009. Natl Vital Stat Rep 2014;62:1-63. [PubMed]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498-504. [Crossref] [PubMed]
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-76. [Crossref] [PubMed]
- Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med 1989;83:195-8. [Crossref] [PubMed]
- Shen KR, Cerfolio RJ. Decision making in the management of secondary spontaneous pneumothorax in patients with severe emphysema. Thorac Surg Clin 2009;19:233-8. [Crossref] [PubMed]
- Passlick B, Born C, Häussinger K, et al. Efficiency of video-assisted thoracic surgery for primary and secondary spontaneous pneumothorax. Ann Thorac Surg 1998;65:324-7. [Crossref] [PubMed]
- Waller DA. Video-assisted thoracoscopic surgery for spontaneous pneumothorax--a 7-year learning experience. Ann R Coll Surg Engl 1999;81:387-92. [PubMed]
- Onuki T, Murasugi M, Ikeda T, et al. Thoracoscopic surgery for pneumothorax in older patients. Surg Endosc 2002;16:355-7. [Crossref] [PubMed]
- Isaka M, Asai K, Urabe N. Surgery for secondary spontaneous pneumothorax: risk factors for recurrence and morbidity. Interact Cardiovasc Thorac Surg 2013;17:247-52. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Ibe T, et al. Surgical treatment for elderly patients with secondary spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2016;64:267-72. [Crossref] [PubMed]
- Shigematsu H, Andou A, Higashi R. Surgical treatment for secondary pneumothorax in patients aged more than 80 years. The Journal of thoracic and cardiovascular surgery 2013;145:880-2. [Crossref] [PubMed]
- Jeon HW, Kim YD, Choi SY, et al. When Is the Optimal Timing of the Surgical Treatment for Secondary Spontaneous Pneumothorax? Thorac Cardiovasc Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- Lippert HL, Lund O, Blegvad S, et al. Independent risk factors for cumulative recurrence rate after first spontaneous pneumothorax. Eur Respir J 1991;4:324-31. [PubMed]
- Zhang Y, Jiang G, Chen C, et al. Surgical management of secondary spontaneous pneumothorax in elderly patients with chronic obstructive pulmonary disease: retrospective study of 107 cases. Thorac Cardiovasc Surg 2009;57:347-52. [Crossref] [PubMed]


