Amide-linked local anesthetics induce apoptosis in human non-small cell lung cancer
Introduction
Local anesthetics have a broad pharmacological roles that go beyond the known analgesia and antiarrhythmic (1). They are used in a wide range of clinical situations to prevent or reduce acute pain, chronic pain and cancer pain (2). A retrospective analysis of patients undergoing cancer surgery suggests that using regional anesthesia may reduce cancer recurrence and improve survival rate (3,4). Recently, some studies have demonstrated that local anesthetics inhibit proliferation, suppress invasion and migration, and induce apoptosis at a range of certain concentrations (5-7). The mechanisms are still unclear. It seems to be unrelated to the sodium-channel blockade (8-10), while in other reports local anesthetics work in the manner of inhibiting the activity of sodium channels (11,12). Previous study has suggested that local anesthetics could induce apoptosis in human thyroid cancer cells, which is associated with mitogen-activated protein kinase (MAPK) pathways (13). Another report has suggested that inhibition of MAPK pathways protects against local anesthetics-induced neurotoxicity (14). However, little is known about local anesthetics-induced cytotoxicity in human non-small-cell lung cancer (NSCLC) cells.
Methods
Cells lines and culture conditions
Human NSCLC cell lines A549 and H520 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science, China. Cells were cultured in a RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% penicillin (100 U/mL) and streptomycin (100 µg/mL) (Gibco, NY, USA). Both cell lines were incubated at 37 °C in a humid incubator with 5% CO2.
MTS assay
Approximately 5×103 cells/well were placed in a 96-well plate and then treated with lidocaine or ropivacaine individually at concentrations ranging from 0 to 32 mM for 24, 48, or 72 h. In each well, 100 µL MTS reagent (Promega, WI, USA) was added, followed by incubated in the dark at 37 °C for 1.5 h. The absorbance values were measured at 490 nm using Varioskan Flash (Thermo Fisher Scientific Inc., MA, USA). The median 50% effective dose (ED50) values were calculated using the probit method of Miller and Tainter.
Cell cycle assay
Cells were exposed to lidocaine or ropivacaine for 24 h at the concentration of 24 h ED50, then washed by phosphate-buffered saline (PBS) and fixed with 75% ethanol overnight at 4 °C. After that, cells were incubated with Rnase (0.1 mg/mL) for 30 min, followed by stained with 40 µg/mL propidium iodide (PI) for another 30 min. Analysis was performed using the Cell Quest software of a Becton-Dickinson fluorescence-activated cell sorting (FACS) Calibur flow cytometer (Becton-Dickinson, CA, USA), the excitation wavelength was set at 488 nm.
Apoptosis assay
Cells were collected after treated with lidocaine or ropivacaine for 24 h, and a minimum of 15,000 cells were analyzed in each measurement. Cells were stained with FITC-conjugated anti-annexin V antibody and PI, then quantified by FACS Calibur flow cytometer with a 488 nm argon laser. The cells in the early state of apoptosis were stained with annexin V, while the late state of apoptosis was stained with PI.
Invasion and migration assays
The upper chamber of a 6.5 mm Transwell polycarbonate membrane were coated with diluted matrigel (3.9 mg/mL, 60–80 µL) inserting with 8 µm pores (Coster, MA, USA). Cells were resuspended in 300 µL serum-free RPMI-1640 medium with no other supplements or with local anesthetic and were incubated for 15 min at room temperature. The inserts were then placed into 500 µL complete medium (RPMI-1640, 10% fetal bovine serum, 1% penicillin and streptomycin plus the same concentration of local anesthetic as present in the upper chamber) in a 24-well plate. After incubation with lidocaine or ropivacaine for 24 h, the upper surface cells were scraped, however, the lower side cells were fixed with 75% ethanol, followed by stained with crystal violet. The migration assay was conducted similarly, with no matrigel on the upper chamber. The inverted microscope was used to count the number of cells at three randomly selected visual fields with 400× magnification.
Detection of intracellular reactive oxygen species (ROS)
The intracellular ROS level was detected using an oxidation-sensitive fluorescent probe. The cells were plated at a density of 3×105 cells/well in 6-well plates and treated with lidocaine or ropivacaine for another 24 h. Subsequently, cells were centrifuged and resuspended in 500 µL of 2,7-dichlorofluorescein diacetate (10 µM, DCFH-DA, Molecular Probes, OR, USA) for ROS detection. After incubated at 37 °C for 30 min, cells from each treatment were analyzed by flow cytometry.
Detection of mitochondrial membrane potential (MMP, ∆Ψm)
MMP was determined by flow cytometry using the ∆Ψm-dependent fluorescent dye JC-1 (Sigma, MO, USA). The cationic dye JC-1 was a highly specific probe for detecting changes in ∆Ψm to evaluate mitochondrial membrane integrity for which could selectively enter into mitochondria and undergo a reversible change in fluorescence emission according to the ∆Ψm. Approximately 3×105 cells/well were cultured in a 6-well plate. After treated with local anesthetic for 24 h, cells were harvested and incubated with JC-1 for 20 min at 37 °C according to the manufacturer’s instructions (Sigma, MO, USA). Then the samples were subjected to ∆Ψm determination by flow cytometry as JC-1 formed red fluorescence in intact mitochondria, while green fluorescence was formed in JC-1 monomers at low ∆Ψm.
Comet assay
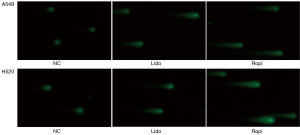
Comet assay was uesd to evaluate the effect of local anesthetics on DNA damage of NSCLC cells. Cells were harvested and resuspended at a density of 1×105/mL to spread on microscopic slides, precoated with a thin layer of 0.5% melting agarose. After gelling for 15 min at 4 °C, slides were incubated in lysis solution for 1.5 h. Afterward, each slide was placed in a tank containing balanced solution for another 20 min. Subsequently, each slide was subjected to electrophoresis at 30 V for 40 min. After immersed in neutralization buffer for 5 min and stained with SYBR Green (Invitrogen, CA, USA), the slides were observed with an FV-1000 laser scanning fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The tails of the comet reflected the DNA damage.
Western blot assay
Equivalent amount of proteins (30 µg) were individually subjected to gel electrophoresis. The proteins were incubated with the indicated primary antibodies: Fas and FasL, Bax and Bcl-2, endonuclease G (Endo G), apoptosis-inducing factor (AIF), cytochrome c, caspase and cleaved caspase-3, -8, -9, poly ADP-ribose polymerase (PARP) and cleaved PARP, cyclin D1, total extracellular signal-regulated protein kinases (ERKs), total c-Jun NH2-terminal kinases (JNKs), total p38 MAPK, p-ERKs, p-JNKs, and p-p38. Anti-β-actin was used as a loading control. Corresponding horseradish peroxidase-conjugated secondary antibodies were used against each primary antibody. Proteins were detected using the chemiluminescent detection reagents.
Statistics analysis
Data were showed as mean ± standard deviation (SD). Statistical analysis of the differences between two groups was evaluated using the one-way analysis of variance, followed by Student’s t-test using the SPSS 16.0 software (SPSS Inc., IL, USA). Values of P<0.05 were considered significant differences (*P<0.05; **P<0.01; ***P<0.001).
Results
Local anesthetics suppressed NSCLC cell viability
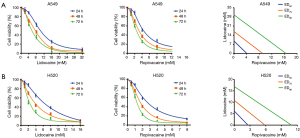
The cell viability assay showed that local anesthetics suppressed the growth of NSCLC cells in a dose- and time-dependent manner (Figure 1). The ED50 values of lidocaine and ropivacaine in A549 cells were higher than that in H520 cells. The ED50 of lidocaine was 9.51 and 6.14 mM for A549 and H520 cells at 24 h, respectively. In addition, the ED50 of ropivacaine at 24 h was 4.06 and 2.62 mM for A549 and H520 cells, respectively.
Local anesthetics arrested NSCLC cell cycle at the G0/G1 phase
As shown in Figure 2A, the percentage of cells in the G0/G1 phase in the treated groups were significantly increased. The cell cycle distribution analysis indicated 91.51%±1.53% A549 and 87.72%±1.60% H520 for lidocaine-treated groups compared with 69.53%±1.62% A549 and 64.94%±1.44% H520 for negative control (NC) groups at the G0/G1 phase (***P<0.001). Simultaneously, ropivacaine-treated groups were 92.57%±1.57% A549 and 86.65%±1.27% H520 at the G0/G1 phase (***P<0.001) (Figure 2A).
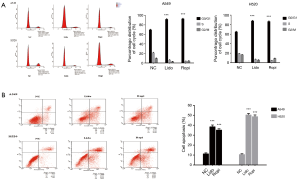
Local anesthetics induced NSCLC cells apoptosis
The total percentage of apoptosis (including early and late apoptosis) was significantly increased in the treated groups (***P<0.001) (Figure 2B).
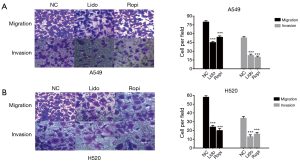
Local anesthetics inhibited invasion and migration of NSCLC cells
The invasion capability of local anesthetics-treated groups decreased in comparison with NC groups, as the number of cells invading through the membrane matrix was obviously decreased (***P<0.001) (Figure 3). Similar to invasion, the migration was also drastically suppressed in local anesthetics-treated groups than in NC groups (***P<0.001) (Figure 3).
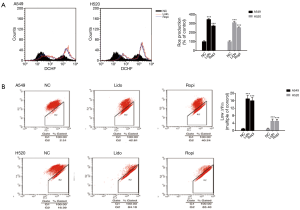
Local anesthetics induced mitochondrion and DNA damage
The level of ROS increased and ∆Ψm decreased (***P<0.001) (Figure 4). In addition, local anesthetics caused oligo nucleosomal DNA fragmentation in NSCLC cells (Figure 5).
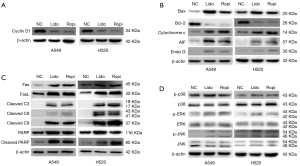
Altered expression of related proteins
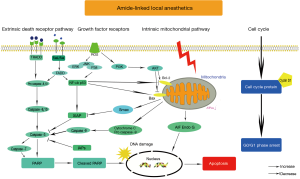
Western blot assay showed that the expression level of cyclin D1 decreased in local anesthetics-treated groups in comparison with NC groups, which was inconsistent with the result of G0/G1 phase arrest (Figure 6A). In addition, the expression level of the intrinsic mitochondrial pathway proteins Bax, Bcl-2, cleaved caspase-9, cytochrome c, AIF and Endo G changed accordingly (Figure 6B,C). Caspase-3 and PARP, the common proteins of two apoptotic pathways, were cleaved after local anesthetics treatment. Furthermore, the expression levels of the extrinsic death receptor pathway proteins Fas and its receptor FasL, cleaved caspase-8 were upregulated (Figures 6C). Moreover, local anesthetics did not alter the expression of p38 MAPK and its phosphorylation (Figure 6D). Unlike p38 MAPK, lidocaine and ropivacaine had no obvious effect on total ERK1/2 and JNK levels; however, the increased phosphorylation of ERK1/2 and JNK were observed (Figure 6D). In total, lidocaine and ropivacaine triggered apoptosis in human NSCLC cells via apoptotic pathways and MAPK pathways (Figure 7).
Discussion
As the leading cause of cancer-related mortality globally, the annual burden of lung cancer is larger than that of any other cancers, for which more than 85% of those cases are currently classified as NSCLC (15-17). Despite recent advances in diagnosis and treatment, the predicted 5-year survival rate is only 15.9% which has only marginally improved during the past decades (18). Thus, the underlying molecular mechanisms and new therapeutic strategies are urgently required in lung cancer.
Retrospective studies of patients undergoing cancer surgeries suggest that using regional anesthesia reduces the risk of tumor metastasis and recurrence, but the mechanism remains unclear (19-21). The benefits may be due to the attenuation of immunosuppression by regional anesthesia (12,22). Some in vitro animal data demonstrate that opioids promote tumor growth and metastasis, largely by inducing mitogenesis and angiogenesis (23,24). Regional anesthesia, in part, reduces the use of opioids, and thus may reduce tumor recurrence and improve survival. However, Doornebal et al. study shows that morphine does not facilitate breast cancer progression (25). Thus, further studies need to be conducted for the specific mechanisms of opioids on cancer. Apart from the preservation of immune system and the reduction in opioids requirement, systemic administration of local anesthetics during surgery plays a role of anti-hyperalgesic and anti-inflammatory (26,27). One paramount benefit of local anesthetics is that they may induce apoptosis in tumor cells but not in normal tissues (23).
The effects of lidocaine and ropivacaine on NSCLC cells in vitro were examined in the present study, as they are the two most commonly used amide-linked local anesthetics in China. Our study demonstrated that lidocaine and ropivacaine inhibited cell growth and arrested cell cycle at G0/G1 phase. Once the cells from the G1 phase moved into the S phase, they could no longer rely on external stimuli, and complete the cell division automatically (28). In all known cell cycle proteins, cyclin D1 was the most critical checkpoint protein in regulating G1 phase to S phase (28). Our study demonstrated that the expression of cyclin D1 was downregulated which could prevent cells move from G1 to S phase thus inhibiting cell growth. The overexpression of cyclin D1 was associated with poor prognosis, and could significantly reduce postoperative long-term survival rate (28). Thus, downregulation the expression and function of cyclin D1 have become one of the important hot areas targeting the drug antitumor research.
Additionally, invasion and migration were suppressed by lidocaine and ropivacaine treatment at a certain range of concentrations which meant the reduction of tumor malignancy.
Furthermore, lidocaine and ropivacaine treatment induced apoptosis. Apoptotic pathways include two major signaling routes: the extrinsic death receptor pathway and the intrinsic mitochondrial pathway (29,30). Apoptosis was mainly controlled by caspases, a family of intracellular cysteine proteases, which were grouped into initiators (caspase-2, -8, -9, and -10) and effectors (caspase-3, -6, and -7) (31,32).
Caspases could activate through being cleaved. Firstly, lidocaine and ropivacaine could activate the extrinsic death receptor pathway. Protein ligand Fas bound to its receptors FasL activating the initiator caspase-8 (31). Moreover, Bcl-2 family participated in the apoptotic process, functioning as promoters (Bax) or inhibitors (Bcl-2). Activated Bax could form an oligomeric pore, resulting in the permeabilization of the mitochondrial outer membrane along with a concomitant decrease in the Bcl-2 level (30,33). An increase of Bax/Bcl-2 ratio could contribute to increased sensitivity of cells to apoptosis. A decrease in ∆Ψm was an early event indicating apoptosis, simultaneously with the increase of Bax/Bcl-2 ratio (30). Lidocaine and ropivacaine downregulated ∆Ψm resulting in mitochondrial dysfunction. The dysfunction of mitochondrion released apoptogenic proteins cytochrome c from mitochondria to the cytosol, resulting in the activation of downstream caspases which was ultimately required to induce apoptosis. Endo G and AIF were also released from mitochondria, and then translocated to the nuclei to induce apoptosis via caspase-independent mitochondrial apoptotic pathway. All in all, these results suggested that local anesthetics could activate the mitochondrial apoptotic pathway (34).
Cleaved caspase-3, the active form of caspase-3, was the capital cleavage enzyme in apoptosis (13). Apoptosis was characterized by the nuclear DNA degradation in response to a variety of apoptotic stimuli (35,36). PARP could be cleaved by caspase-3 and -7 during apoptosis which was involved in DNA damage and repair. This cleavage inactivated PARP contributed to cells’ apoptosis (8). Increased PARP cleavage was observed in NSCLC cells after treated with lidocaine or ropivacaine.
In addition to the two classical apoptotic pathways, ROS production was upregulated, which was an explicit indicator of apoptosis (34). The increased ROS production was a clear indication of apoptosis via activating endoplasmic reticulum (ER) stress pathway, which included MAPK pathways (34). The members of MAPK family, including ERKs, JNKs, and p38 MAPK, were activated by phosphorylation on threonine and tyrosine residues by upstream dual-specificity kinases (37). The results showed the phosphorylation of ERK1/2 and JNK increased, suggesting that ERK1/2, JNK, and p38 MAPK may have different effects on local anesthetics induced NSCLC cells apoptosis.
In summary, local anesthetics affect the outcomes of NSCLC in a variety of aspects, including arrest cell cycle, induce apoptosis, and inhibit invasion and migration. In addition, local anesthestics may attenuate the neuroendocrine response due to surgery, thus improve the preservation of immunocompetence. Furthermore, local anesthetics may make tumor cells more sensitive to the effects of chemotherapy. Taking into account that local anesthetics used for postoperative pain relief specially via intrapleural analgesia after minimally invasive thoracoscopic surgery (38,39), our study indicate the additional benefits of local anesthetics in lung cancer surgery which may have substantial clinical implications.
Conclusions
Our study indicates that amide-linked lidocaine and ropivacaine trigger apoptosis in human NSCLC cells via apoptotic pathways and MAPK pathways. The results reveal the beneficial actions of amide-linked local anesthetics and call for further studies to their use during lung cancer surgery.
Acknowledgements
Funding: This study was supported by grants from the Scientific Research Project of Health and Family Planning Commission of Zhejiang Province, China (No. 201475562, 2015108467 and 2016147673).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Borgeat A, Aguirre J. Update on local anesthetics. Curr Opin Anaesthesiol 2010;23:466-71. [Crossref] [PubMed]
- Heavner JE. Local anesthetics. Curr Opin Anaesthesiol 2007;20:336-42. [Crossref] [PubMed]
- Tavare AN, Perry NJ, Benzonana LL, et al. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer 2012;130:1237-50. [Crossref] [PubMed]
- Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 2010;105:106-15. [Crossref] [PubMed]
- Kobayashi K, Ohno S, Uchida S, et al. Cytotoxicity and type of cell death induced by local anesthetics in human oral normal and tumor cells. Anticancer Res 2012;32:2925-33. [PubMed]
- Cassinello F, Prieto I, del Olmo M, et al. Cancer surgery: how may anesthesia influence outcome? J Clin Anesth 2015;27:262-72. [Crossref] [PubMed]
- Lucchinetti E, Awad AE, Rahman M, et al. Antiproliferative effects of local anesthetics on mesenchymal stem cells: potential implications for tumor spreading and wound healing. Anesthesiology 2012;116:841-56. [Crossref] [PubMed]
- Chang YC, Liu CL, Chen MJ, et al. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg 2014;118:116-24. [Crossref] [PubMed]
- Gao Z, Xu Z, Hung MS, et al. Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol Rep 2009;22:1479-84. [PubMed]
- Piegeler T, Votta-Velis EG, Liu G, et al. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology 2012;117:548-59. [Crossref] [PubMed]
- Baptista-Hon DT, Robertson FM, Robertson GB, et al. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth 2014;113 Suppl 1:i39-i48. [Crossref] [PubMed]
- Fraser SP, Foo I, Djamgoz MB. Local anaesthetic use in cancer surgery and disease recurrence: role of voltage-gated sodium channels? Br J Anaesth 2014;113:899-902. [Crossref] [PubMed]
- Chang YC, Hsu YC, Liu CL, et al. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One 2014;9:e89563. [Crossref] [PubMed]
- Lirk P, Haller I, Colvin HP, et al. In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesth Analg 2008;106:1456-64. table of contents. [Crossref] [PubMed]
- Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin 2011;61:91-112. [Crossref] [PubMed]
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727-40. [Crossref] [PubMed]
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. [Crossref] [PubMed]
- Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: recent developments. Lancet 2013;382:709-19. [Crossref] [PubMed]
- Hiller J, Ismail H, Riedel B. Improved quality of anesthesia and cancer recurrence studies. Anesth Analg 2014;119:751-2. [Crossref] [PubMed]
- Fodale V, D'Arrigo MG, Triolo S, et al. Anesthetic techniques and cancer recurrence after surgery. ScientificWorldJournal 2014;2014:328513.
- Cata JP, Gottumukkala V, Thakar D, et al. Effects of postoperative epidural analgesia on recurrence-free and overall survival in patients with nonsmall cell lung cancer. J Clin Anesth 2014;26:3-17. [Crossref] [PubMed]
- Mao L, Lin S, Lin J. The effects of anesthetics on tumor progression. Int J Physiol Pathophysiol Pharmacol 2013;5:1-10. [PubMed]
- Durieux ME. Anesthesia and cancer recurrence: improved understanding, but no reason for change. Anesth Analg 2014;118:8-9. [Crossref] [PubMed]
- Niwa H, Rowbotham DJ, Lambert DG, et al. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? J Anesth 2013;27:731-41. [Crossref] [PubMed]
- Doornebal CW, Vrijland K, Hau CS, et al. Morphine does not facilitate breast cancer progression in two preclinical mouse models for human invasive lobular and HER2+ breast cancer. Pain 2015;156:1424-32. [Crossref] [PubMed]
- Xuan W, Hankin J, Zhao H, et al. The potential benefits of the use of regional anesthesia in cancer patients. Int J Cancer 2015;137:2774-84. [Crossref] [PubMed]
- Votta-Velis EG, Piegeler T, Minshall RD, et al. Regional anaesthesia and cancer metastases: the implication of local anaesthetics. Acta Anaesthesiol Scand 2013;57:1211-29. [Crossref] [PubMed]
- Lin DI, Lessie MD, Gladden AB, et al. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene 2008;27:1231-42. [Crossref] [PubMed]
- Matthews GM, Newbold A, Johnstone RW. Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity. Adv Cancer Res 2012;116:165-97. [Crossref] [PubMed]
- Brinkmann K, Kashkar H. Targeting the mitochondrial apoptotic pathway: a preferred approach in hematologic malignancies? Cell Death Dis 2014;5:e1098. [Crossref] [PubMed]
- Xu H, Zhao X, Liu X, et al. Antitumor effects of traditional Chinese medicine targeting the cellular apoptotic pathway. Drug Des Devel Ther 2015;9:2735-44. [PubMed]
- Khan KH, Blanco-Codesido M, Molife LR. Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev Oncol Hematol 2014;90:200-19. [Crossref] [PubMed]
- Bates DJ, Lewis LD. Manipulating the apoptotic pathway: potential therapeutics for cancer patients. Br J Clin Pharmacol 2013;76:381-95. [Crossref] [PubMed]
- Yu CC, Ko FY, Yu CS, et al. Norcantharidin triggers cell death and DNA damage through S-phase arrest and ROS-modulated apoptotic pathways in TSGH 8301 human urinary bladder carcinoma cells. Int J Oncol 2012;41:1050-60. [PubMed]
- Liu D, Shi P, Yin X, et al. Effect of norcantharidin on the human breast cancer Bcap-37 cells. Connect Tissue Res 2012;53:508-12. [Crossref] [PubMed]
- Shen B, He PJ, Shao CL. Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLoS One 2013;8:e84610. [Crossref] [PubMed]
- Zhu W, Zou Y, Aikawa R, et al. MAPK superfamily plays an important role in daunomycin-induced apoptosis of cardiac myocytes. Circulation 1999;100:2100-7. [Crossref] [PubMed]
- Ishikawa Y, Maehara T, Nishii T, et al. Intrapleural analgesia using ropivacaine for postoperative pain relief after minimally invasive thoracoscopic surgery. Ann Thorac Cardiovasc Surg 2012;18:429-33. [Crossref] [PubMed]
- Inoue S, Nishimine N, Furuya H. Unintentional intrapleural insertion of an epidural catheter: should we remove it or leave it in situ to provide perioperative analgesia? Anesth Analg 2005;100:266-8. [Crossref] [PubMed]


