|
Systemic neoadjuvant chemotherapy:evolution, major dilemmas and points
of interest
History and early trials
The first prospective study for NCT in locally advanced,
inoperable breast cancer is dated in 1973, by the European
Institute of Oncology and the primary purpose was to downstage
the primary tumor in order to achieve surgical resection ( 15).
Many other trials followed in the past two decades studying the
role of induction chemotherapy. Currently NCT followed by
surgery, is the treatment of choice for patients with IBC or LABC
( 16, 17). Recently this approach was also recommended for
primary operable disease ( 18). The early 80’s and 90’s trials that evaluated the role of NCT
highlighted the potential of this treatment approach. These
trials concluded survival improvement up to 25% at 10 years of
follow up. These studies focused on anthracycline based or CMF
[Cyclophosphamide- Methotrexate- 5-fluorouracil (5-FU)]-
like regimens, compared with historical experience on local
therapy alone. However, early trials were highly heterogeneous in
many aspects. In fact, they included heterogeneous populations
in regard with the stage of the disease. They usually included
advanced together with earlier stages of operable breast
cancer (OBC). They mostly used CMF-like and anthracycline
containing regimens but also radiotherapy (RT) and rarely
endocrine therapy. In addition, differences in defining
operability especially in the 80’s rendered even more difficult
the comparison of groups studied with historical controls.
Moreover, the majority of these studies were of small size, mostly
non-randomized and did not report the long-term impact of
the neoadjuvant approach on multiple outcomes, including
survival. As a result, while highlighting the potential of induction
chemotherapy in the treatment of breast cancer, these studies
illustrated many of the difficulties associated with the evaluation
of NCT benefits.
Despite multiple discrepancies, these early trials established
a solid background for the development of randomized studies.
This allowed a better determination of the long-term impact of
this treatment approach and its relative benefit compared with
adjuvant therapy. Consequently, we will attempt to address
and present important questions and points of interest in the
neoadjuvant treatment research in LABC, based on results from
well established randomized trials and also with referral to this
early pool of data.
Neoadjuvant vsadjuvant systemic
chemotherapy
One of the early concerns has been the validation of NCT against
adjuvant systemic therapy. Data in locally advanced disease are
limited mainly due to the lack of randomized trials comparing
neoadjuvant to adjuvant chemotherapy. In part this happened because many surgeons consider these tumors inoperable prior
to chemotherapy. Contrarily, there is a large body of randomized
trials in OBC. In these studies a minority of women with LABC
is also enrolled.
In fact, two large randomized trials of National Surgical
Adjuvant Breast and Bowel Project (NSABP) addressed the
question of neoadjuvant versus (vs) adjuvant chemotherapy
but both in OBC patients. In the NSABP B-18 trial ( 19), 1,523
women with primary OBC were randomly assigned to four
cycles of doxorubicin (A) and cyclophosphamide (C) either
prior or following surgery. No significant difference in OS among
the two groups was noted, with a median follow-up of 9 years.
However, women achieving a pCR had a 50% reduction in risk
of death compared to the entire group. The larger NSABP B-27
trial ( 20), with 2,411 patients, evaluated the addition of a taxane
following AC either in neoadjuvant or adjuvant setting in a three
arm design: a) AC and then surgery, b) AC plus taxane and then
surgery, c) AC, surgery and then adjuvant chemotherapy with a
taxane. The addition of docetaxel (DOC) pre- or post- surgery
also made no significant difference. Despite the fact that the
pCR rate was almost doubled from 13.7% in the NSABP B-18,
to 26.1% in B-27, a significant OS difference was not observed
between the treatment arms. However patients with pCR had
improved OS (HR = 0.33, P <0 .0001) and DFS (hazard ratio
[HR] = 0.45, p < 0.0001), at 6.5 years of follow-up, confirming
that pCR can be used as a surrogate marker for improved longterm
prognosis. The results of NSABP B18 and B27 with an
extended follow-up of 16 and 8.5 years’ respectively have also
been published ( 21). In both protocols the results demonstrate
that preoperative therapy is equivalent to adjuvant therapy and
there are no statistically significant differences in OS and DFS.
Hence, in NSABP B-18 trial there were trends in favor of preoperative
chemotherapy for disease-free survival (DFS) and OS
in women less than 50 years old. In addition, preoperative DOC
added to AC significantly increased the proportion of patients
having pCRs compared with preoperative AC alone (26% vs
13%, P< 0.0001). In both studies, patients who achieved a pCR
continue to have significantly superior OS and DFS ( 21). Many other studies compared neoadjuvant and adjuvant
chemo-endocrine therapies ( 22, 23), including different
regimens in pre- and post- operative setting ( 24-26). In general,
comparable results appear with either approach and with no
overall substantial differences. A meta-analysis that was published
in 2005 by Mauri et al. ( 27) included nine randomized studies.
They compared neoadjuvant vs adjuvant therapy, regardless
of regimen and local therapy. In accordance with all previous
trials, this meta-analysis did not reveal substantial difference
between the two treatment settings in disease progression,
distant recurrence rates or mortality. On the other hand, NCT
was associated with an increased risk of loco-regional recurrence
compared to adjuvant therapy. The conclusion was that NCT and adjuvant chemotherapy had equivalent rates of survival and
disease progression. Dan Costa et al. ( 28) performed a secondary analysis of the
GeparTrio trial Data. NCT shows similar response in patients
with OBC and LABC/IBC. In this study although response rates
(RR), clinical (c) and pCR presented significant differences in
the subgroups, in multivariable analysis tumor stage was not
an independent predictor for pCR. At least to our knowledge,
none of the other trials in NCT, provides a direct comparison
of LABC/IBC with OBC results. Data from this study provide
for the first time, direct evidence for similar response patterns
throughout all stages of breast cancer. Based on this observation
one might attempt to extrapolate results of the OBC trials to
LABC populations ( 28). Summarizing, these trials have shown that NCT is well
tolerated and significantly improves outcomes compared to
surgery alone, with at least comparable results to those of
adjuvant chemotherapy. The latter has largely contributed to the
establishment of NCT as part of standard treatment in patients
with LABC.
The critical question: which regimen and for
how long?
The choice of the optimal chemotherapy regimen and the
duration of treatment have been extensively assessed in induction
systemic chemotherapy but no consensus has been developed so
far. Beyond the pivotal data from early anthracycline and CMFlike
containing studies of NCT, more recent randomized trials in
LABC focus on the addition of newer agents. All these trials are
based to well established regimens used in the adjuvant setting
research.
The study of Hutcheon et al. ( 29) was one of the early phase
III randomized trials in LABC patients that confirmed the
superiority of the sequential neoadjuvant approach of 4 cycles
of an anthracycline regimen followed by 4 cycles of DOCbased
regimen. This regimen was compared to 8 cycles of an
anthracycline regimen alone. A high number of relative trials
evaluated the role of different combinations of anthracyclines
and taxanes. The Aberdeen Breast Study Group has conducted
a two arm randomized trial ( 30, 31) that compared eight cycles
of neoadjuvant cyclophosphamide, vincristine, doxorubicin
and prednisone (CVAP) vs four cycles of neoadjuvant CVAP
followed by four cycles of neoadjuvant DOC. Significantly
greater RRs (85% vs 64%), pCR rates (31% vs 15%), 5 year OS
(93% vs 78%) and also incidence of breast-conserving surgery
(67% vs 48%), were observed for patients in the DOC containing
arm. This result suggests that the use of sequential, non-crossresistant
chemotherapeutic agents, such as anthracyclines and
taxanes, can improve survival. Instead, pCR was only 2% in the
subgroup of patients with initially stable or progressive disease to
CVAP that then switched to DOC. This suggests that initial no responders do not benefit from switching to another regimen. The study of Evans et al. ( 32), a well designed randomized
trial, compared the clinical and pathologic RR of AC vs
doxorubicin and docetaxel (AT), as primary chemotherapy
in women with LABC. In contrast to the positive results
reported for the sequential use of DOC after AC as induction
chemotherapy, this study did not suggest a benefit for
simultaneous AT over AC. However, encouraging were the
results of a small non randomized trial in stage III breast cancer
patients treated with NCT. Four cycles of DOC single therapy
resulted in a 7% pCR rate in the breast and axilla (95% CI 2%
to 21%) and a 5-year overall survival rate of 80% ( 33). Another
randomized trial of Untch et al. ( 34), evaluated the role of a
dose-dense sequential schedule of epirubicin (E) and paclitaxel
(PAC). A significantly higher frequency of breast conserving
surgery and a higher pCR rate were observed as compared to E/
PAC in standard dose. With a similar design in the GEPAR-DUO study ( 35), the
dose-dense combination of A plus DOC administered for four
cycles has resulted to a clinical RR of 73% and pCR of 7%.
Preliminary results, from a small phase II trial ( 31), with the
use of a neoadjuvant DOC and vinorelbine (V) regimen appear
promising, with an RR of 100%, cCR rate of 59% and pCR rate
of 31%. Another recent report, evaluates the activity and safety of
a non anthracycline-regimen, containing PAC plus carboplatin,
in 107 patients with bilateral breast cancer ( 36). Clinical RR was
86.1% (CR: 32.4%) while twenty-one patients achieved pCR
(19.4%). Many other phase II and III trials have studied the role of
taxanes in pre-operative setting. These trials have been highly
heterogeneous with different patient populations. They often
included operable and inoperable disease and a mixture of nodepositive
and node-negative patients. Furthermore, regimen
selections varying between sequential and combination
schedules, with different number of cycles. However, the use of
taxanes in the induction therapy setting seems to be associated
with improved outcomes in various endpoints assessed ( 37-44).
In these studies sequential administration of an anthracycline
and DOC in the neoadjuvant setting resulted in clinical RRs
ranging from 85% to 93% and pCR rates from 11% to 31%. For
anthracycline and DOC in combination setting, clinical RRs and
pCR rates range from 68% to 93% and 8% to 16%, respectively.
Other non taxane-containing regimens and alternative
schedules have been tested in NCT. Of special interest is the
phase III randomized study of Therasse et al. ( 45). In this study
a preoperative anthracycline-based regimen was compared with
a similar regimen with dose intensification. The dose dense arm
was not superior. It should be noted that due to discrepancies
between the two arms the interpretation of the results is difficult
and no safe conclusion could be extracted. Another promising approach is that of metronomic dose scheduling in neoadjuvant setting. A phase III study of Ellis et al.
( 46) (SWOG trial 0012) evaluated this approach. In this study,
265 patients with LABC were randomized to conventional AC vs
metronomic dosing of A, 24 mg/m 2 weekly, and C, daily oral 60
mg/m 2 plus growth factor-support for 15 weeks and then both
arms followed by standard weekly PAC for 12 weeks. Preliminary
results of this trial suggest an improved pCR rate (19% vs
31%, respectively; OR=2.11, P=0.020) with the metronomic
schedule. The pCR improvement was most pronounced in
the inflammatory cohort (12% vs 32%). Many other different
regimens have been tested with positive results, although no
definitive advantage has been demonstrated for any of them
( 47-55). Moreover, the duration of induction chemotherapy remains
an unresolved issue and no optimal approach has been
established. The maximal response to NCT vary widely; there
are patients who achieve maximal tumor reduction after only one
or two cycles, while others require up to eight or more cycles of
treatment, as evidenced from cumulating clinical experience.
The optimal duration of induction treatment with concurrent
taxane and anthracycline containing regimen was also addressed
in the phase III randomized GeparTrio trial ( 56). In this study,
2,090 women with advanced disease were initially treated
with two courses of docetaxel, adriamycin, cyclophosphamide
(TAC). Responders were then randomly assigned to four
versus six additional cycles of TAC, while non responders
were assigned to four additional cycles of TAC or to crossover
to a non anthrcycline regimen with vinorelbine (V) plus
capecitabine (CAP). Among responders, those who received
eight courses of TAC had significantly higher c RR, but the pCR
rates were comparable: 24% vs 21% for eight and six cycles,
respectively. These data suggest that more than six cycles of TAC
do not improve RR, although OS was not addressed. Few more
randomized trials have been undertaken in an attempt to set cut
off point ( 57, 58). Summarizing, until the era of taxanes the common practice
was the use of an anthracycline-based regimen for a minimum
of three to four cycles. Additional courses of the same regimen
administered until reaching a “plateau” of maximal clinical
response and frequently continued for two cycles beyond this
clinical cut off point. This approach was felt to maximize the rate
of complete remission. However, as noted above, emerging data
suggest that both responders and non responders to four cycles
of an initial anthracycline-based regimen benefit from crossover
to a non cross-resistant therapy, usually a taxane and the trend
is to administer the most of systemic chemotherapy before the
local treatment.
Utility of initial response: an important
predictive factor?
One more issue that was subject of extensive debate in NCT is the importance of response to initial chemotherapy. This variable
is an established key criterion of the early era of induction
chemotherapy trials. It represents the main advantage of
preoperative therapy, which is the feasibility to monitor tumor
response and to tailor subsequent treatment based on response.
Nevertheless, no strong correlation of clinical and pathologic
responses has been demonstrated ( 11). Although early clinical response is associated with higher
rates of pCR and better long-term outcome ( 59), trials have not
verified an outcome improvement when treatment planning is
based on clinical response ( 11). In the GeparTrio ( 56) and the
Aberdeen ( 60) trials the subgroup of patients with clinically
non responding disease presented low pCR rate. That was not
consistently improved by switching to a non- cross-resistant
chemotherapy regimen. Although both studies used a similar
randomization (continue treatment or switch to a new regimen),
they were different in the randomization timing (4 vs 2 cycles)
and disease characteristics (sensitive vs resistant disease).
Nevertheless, both studies suggested that the treatment plan
should not be altered based on early response. Unless there is
a clear evidence of disease progression, deviations from the
planned therapy in clinical non responders do not increase
either pCR or clinical response rate (cRR) nor improve survival
( 56, 60). However, in the early NSABP B-27 trial, subgroup
analysis in those patients who had a clinical partial response
after AC indicates that there was a significant DFS benefit when
adding four cycles of preoperative DOC to AC. Of different design was the study by Thomas et al. ( 61), which
evaluated the benefit of adding adjuvant chemotherapy when
NCT does not induce a pCR. In this trial, 193 patients with
LABC received neoadjuvant CAVP and had a c RR of 83.4% and
a pCR rate of 12.2%. The patients, who did not achieve pCR,
were randomized to receive additional CAVP or vinblastine,
methotrexate, leucovorin, and fluorouracil (VbMF). Diseasefree
and OS rates were not statistically different between the two
groups, suggesting little benefit to postoperative chemotherapy
when NCT does not induce a pCR. In summary, pCR to NCT has been consistently associated
with improved DFS and OS and early clinical response usually
correlates with high probability for pCR. On the other hand,
even data extracted from several trials that evaluated early clinical
response as an important parameter, we should be cautious when
it is used as a criterion for early or mid-treatment modulations.
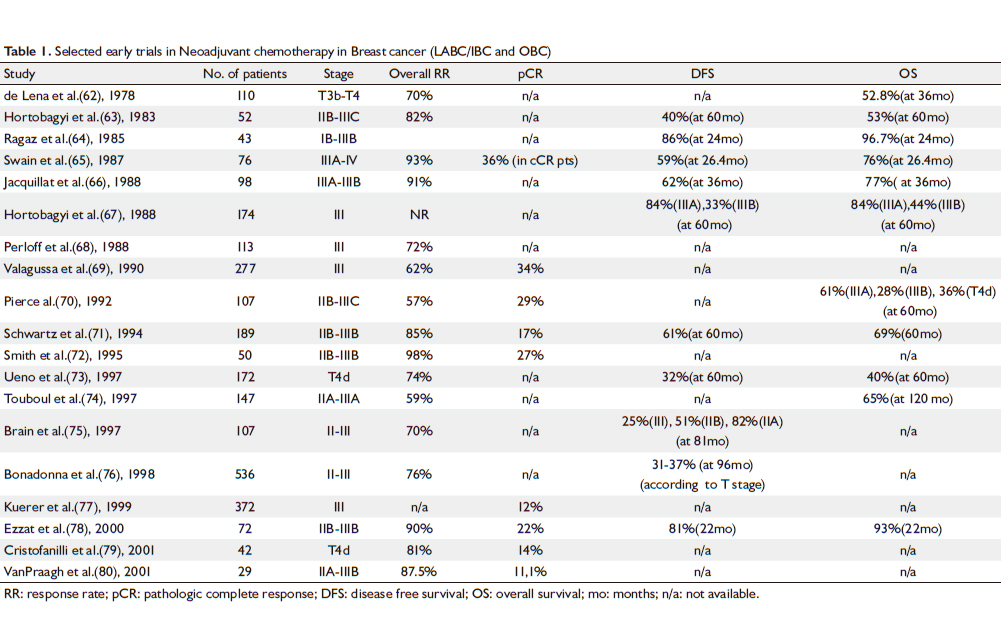
Some of the most important early trials are summarized in
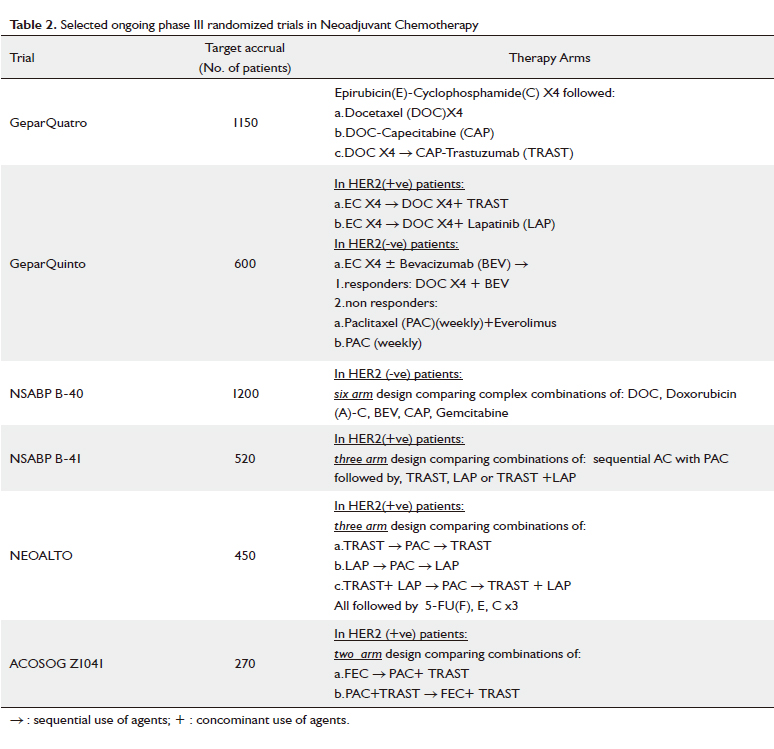
Table 1 ( 62-80) and furthermore, selected ongoing phase III
randomized trials in NCT are presented in Table 2.
Neoadjuvant targeted therapy regimen
The main pathways undergoing therapeutic targeting are
currently HER-2 and angiogenesis. For HER-2 targeting
therapies already exist enough data reported from phase III trials. On the contrary, data on anti-angiogenetic agents are limited so
far.
a. HER-2 targeting therapy
Trastuzumab (TRAST) is a humanized monoclonal anti-
HER2 antibody approved for the treatment of HER2-positive
(+ve) breast cancer either in the adjuvant or metastatic setting.
It is used either as a single agent after chemotherapy or in
combination with chemotherapy. Several trials have examined
the potential benefits of neoadjuvant TRAST combined with
chemotherapeutic agents in patients with HER2+ve tumors
( 81-83). In this review, we are going to focus only to phase III
trials presented so far. Buzdar and colleagues ( 84) conducted a phase III trial to
assess NCT consisting of PAC, 5-FU, E and C with or without
TRAST. The pCR rate was 65.2% in the TRAST arm vs 26.3%
in the chemotherapy-only arm (P=0.016). The 3-year DFS rate
(100% vs 85.3%, respectively) improved with TRAST addition.
Furthermore, in the NOAH (neoadjuvant Herceptin) trial ( 85),
patients with HER2+ve locally advanced or inflammatory breast
cancer were randomized to CT (A, PAC, CMF-regimens) with
or without TRAST. In addition, a subgroup of patients with
HER2-negative(-ve) disease treated with the same chemotherapy
combination was used as control. TRAST significantly improved
event-free survival in patients with HER2+ve breast cancer
in 3 years [71% (95% CI: 61-78) with TRAST vs 56% (95% CI:46-65) without]. Overall response rate was 87% in the
TRAST arm vs 74% without TRAST (P=0.009). Response rates
did not differ in patients with HER2+ve disease who were not
treated with TRAST compared to those with HER2-ve disease.
In the GeparQuattro study ( 86), four cycles of EC followed
by four cycles of DOC with or without CAP and TRAST were
administrated to patients with operable or locally advanced
HER2+ve tumors. Furthermore, a subgroup of patients with
HER2-ve disease was treated with the same chemotherapy
regimen. In HER-2+ve disease patients, pCR was 31.7% vs
15.7% in HER2-ve group of patients. Despite the high pCR rate,
TRAST in patients with HER2+ve disease did not result in a
higher rate of breast-conserving surgery. TRAST in previously described studies was well tolerated
and chronic heart failure rates reported were <1%. Patients who
are candidates for NCT have a high probability to achieve a pCR
if they have HER2+ve tumors. Application of TRAST should be
considered to improve clinical and pathological tumor RR and
outcome.
Lapatinib (LAP), an oral agent that inhibits HER1 and
HER2 receptor tyrosine kinase, is already approved for use
in HER2+ve metastatic breast cancer after progression on
anthracyclines, taxanes, and TRAST. A phase II trial of LAP in
refractory/relapsed IBC reported a c RR of 50% on skin lesions
and a 28% overall RR ( 87). Cristofanilli et al. ( 88) studied LAP
monotherapy followed by LAP and weekly PAC in patients
with newly diagnosed IBC. A c RR of 77% and a pCR of 17% in
patients with HER2+ve IBC were reported. b. Anti - angiogenesis treatment
Bevacizumab (BEV) is a recombinant, humanized, monoclonal
anti- VEGF antibody that targets angiogenesis, vascular
permeability, and endothelial cell growth. Its synergy and
efficacy with other chemotherapeutic agents in metastatic breast
cancer was studied in preclinical and phase I, phase II studies but
also confirmed in phase III trials ( 89-94). Data with BEV in the
neoadjuvant setting are limited to date. In 2004, a phase II trial of neoadjuvant DOC with or without
BEV in LABC was presented. Five CR and 24 PR were observed
( 95). Wedam et al. ( 96) reported on 21 patients with LABC
treated with BEV on cycle 1 and ADOC and BEV for 6 more
cycles. A c RR of 67% (95% CI: 43% - 85.4%) with a 5% pCR
rate was observed. In addition, a median decrease of 66.7% in
phosphorylated VEGFR2 in tumor cells (P=0.004) and increase
of 128.9% in tumor apoptosis (P =0.0008) were seen after BEV
alone. Furthermore, these results persisted with the addition of
chemotherapy. Hurvitz et al. ( 97) reported on a multicenter phase II trial of
neoadjuvant single-agent BEV or placebo, followed by TAC, with
or without BEV, in patients with stage II or stage III breast cancer
[Arm A: TAC + low-dose BEV (7.5 mg/kg); Arm B: TAC + lowdose placebo; Arm C: TAC + standard dose BEV (15 mg/kg);
Arm D: TAC + standard-dose placebo]. 90 patients were initially
enrolled. Of the 37 post- surgery patients, clinical CR rate was
59% (5/12 Arm A; 7/11 Plac- Arms B/D; 10/14 Arm C) and
35% clinical PR (7/12 Arm A; 3/11 Arms B/D; 3/14 Arm C).
In a phase II pilot study ( 98) in HER2-ve patients DOC, CAP
and BEV combination was evaluated. pCR rate was 22% (95%
CI: 6-48). Nine of the patients without pCR achieved clinical
partial response, giving a 72% overall clinical RR (95% CI:
47-90). Waintraub and colleagues ( 95) presented their results
of neoadjuvant dose-dense BEV plus DOC followed by a BEVAC
regimen in HER2-ve LABC. Fifteen patients were enrolled
and of the first 12 post-operative evaluable patients the results
showed 5 pCR (42% pCR rate). In a multicenter pilot study
Yardley et al. ( 100) presented results of weekly nab-paclitaxel,
carboplatin, BEV and TRAST as neoadjuvant therapy in HER2-
ve LABC; pCR was noted in 13/20 patients (65%) and PR was
noted in 7/20 patients (35%). It is obvious that available data are scarce so far and large
prospective phase III trials are warranted to evaluate BEV’s
and other antiangiogenic agents’, as sunitinib and sorafenib,
efficacy in the neoadjuvant setting. Preliminary safety data
are recently reported by GeparQuinto study on BEV or
everolimus or LAP addition to anthracycline- and taxanebased
neo-adjuvant chemotherapy regimens in primary
breast cancer. Adding BEV and everolimus to chemotherapy
appeared feasible, meanwhile it is suggested a decrease of
LAP dose to 1000 mg daily ( 101). c. Preliminary data of attractive agents
Preclinical and preliminary clinical data indicate that Zolendronic
acid through farnesyl diphosphate synthase inhibition has both
direct and indirect antitumour effects in breast cancer ( 102).
In the adjuvant setting, its addition to endocrine treatments
seems to improves outcomes in pre-menopausal women with
early breast cancer ( 99). Performing a retrospective evaluation
within AZURE (Adjuvant Zoledronic acid redUce REcurrence)
trial the clinical data extracted suggested that the addition of
Zolendronic acid to neoadjuvant CT may improve pathological
response ( 104). In vitro and in vivo data support a role of insulin in
carcinogenesis. Metformin, an oral antidiabetic agent that
increases insulin sensitivity and reduces insulin levels was studied
in a retrospective analysis in combination with neo-adjuvant
chemotherapy. A pCR rate of 24% in the combination arm
compared to 8% in patients who were not receiving metformin
was observed (P =0.02) ( 105).
|
|
References
- Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol 2002;20:3628-36.[LinkOut]
- National Cancer Institute, DCCPS, Surveilance Research Program Cancer Statistics Branch (2001). SEER Program Public Use Data Tapes 1973 -1998.
- Buzdar AU, Valero V, Theriault RL, Frye D, Green M, Booser D, et al. Pathological complete response to chemotherapy is related to hormone receptor status [abstract]. Breast Cancer Res Treat 2003;85:2.
- Bonnefoi H, Diebold-Berger S, Therasse P, Hamilton A, van de Vijver M, MacGrogan G, et al. Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: are there molecular markers in the primary tumour that predict for 5 year clinical outcome? Ann Oncol 2003;14:406-13.[LinkOut]
- Gogas H, Pectasides D, Kostopoulos I, Lianos E, Skarlos D, Papaxoinis G, et al. Paclitaxel and carboplatin as neoadjuvant chemotherapy in patients with locally advanced breast cancer: A phase II Trial of the Hellenic Cooperative Oncology Group. Clin Breast Cancer 2010;10:230-7.[LinkOut]
- Kountourakis P, Missitzis I, Doufexis D, Zobolas V, Pissakas G, Arnogiannaki N, et al. Neoadjuvant sequential epirubicin and docetaxel followed by surgery-radiotherapy and post-operative docetaxel or gemcitabine/vinorelbine combination based on primary response: a multimodality approach for locally advanced breast cancer. J Cancer Res Clin Oncol 2010 Apr 13. [Epub ahead of print][LinkOut]
- Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol 2006;24:1037-44.[LinkOut]
- Honkoop AH, van Diest PJ, de Jong JS, Linn SC, Giaccone G, Hoekman K, et al. Prognostic role of clinical, pathological and biological characteristics in patients with locally advanced breast cancer. Br J Cancer 1998;77:621-6.[LinkOut]
- Hortobagyi GN. Comprehensive management of locally advanced breast cancer. Cancer 1990;66:1387-91.[LinkOut]
- Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: A review. J Clin Oncol 1992;10:1014-24.[LinkOut]
- Specht J, Gralow JR. Neoadjuvant chemotherapy for locally advanced breast cancer. Semin Radiat Oncol 2009;19:222-8.[LinkOut]
- Liu SV, Melstrom L, Yao K, Russell CA, Sener SF. Neoadjuvant therapy for breast cancer. J Surg Oncol 2010;15:101:283-91.[LinkOut]
- Mamounas EP. Neoadjuvant chemotherapy for operable breast cancer: is this the future? Clin Breast Cancer 2003;4:s10-9.[LinkOut]
- Sinclair S, Swain SM. Primary systemic chemotherapy for inflammatory breast cancer. Cancer 2010;116:s2821-8.[LinkOut]
- Bonadonna G. Evolving concepts in the systemic adjuvant treatment of breast cancer. Cancer Res 1992;52:2127-37.[LinkOut]
- Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol 2008;26:786-90.[LinkOut]
- Cristofanilli M, Valero V, Buzdar AU, Shu-Wan Kau, Broglio KR, Gonzalez-Angulo AM, et al. Inflammatory breast cancer (IBC) and patterns of recurrence: Understanding the biology of a unique disease. Cancer 2007;110:1436-44.[LinkOut]
- Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: An update. J Clin Oncol 2006;24:1940-9.[LinkOut]
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst 2001;30:96-102.[LinkOut]
- Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project protocol B-27. J Clin Oncol 2006;24:2019-27.[LinkOut]
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-85.[LinkOut]
- Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 1998;9:1179-84.[LinkOut]
- Gazet JC, Ford HT, Gray R, McConkey C, Sutcliffe R, Quilliam J, et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Ann Oncol 2001;12:685-91.[LinkOut]
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672-85.[LinkOut]
- Scholl SM, Asselain B, Palangie T, Dorval T, Jouve M, Garcia Giralt E, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer 1991;27:1668-71.[LinkOut]
- Broet P, Scholl SM, de la Rochefordiere A, Fourquet A, Moreau T, De Rycke Y, et al. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: an updated analysis of a randomized trial. Breast Cancer Res Treat 1999;58:151-6.[LinkOut]
- Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst 2005;97:188-94.[LinkOut]
- Dan Costa S, Loibl S, Kaufmann M, Zahm DM, Hilfrich J, Huober J, et al. Neoadjuvant chemotherapy shows similar response in patients with inflammatory or locally advanced breast cancer when compared with operable breast cancer: a secondary analysis of the GeparTrio trial data. J Clin Oncol 2010;28:83-91.[LinkOut]
- Hutcheon AW, Heys SD, Sarkar TK. Neoadjuvant docetaxel in locally advanced breast cancer. Breast Cancer Res Treat 2003;79:s19-24.[LinkOut]
- Hutcheon AW, Heys SD, Miller ID. Improvements in survival in patients receiving primary chemotherapy with docetaxel for breast cancer: A randomised controlled trial [abstract]. Breast Cancer Res Treat 2001;69:298.
- Hutcheon AW, Heys SD, Sarkar TK. Docetaxel primary chemotherapy in breast cancer: a five year update of the Aberdeen trial [abstract]. Breast Cancer Res Treat 2003;82:s9.
- Evans TRJ, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, et al. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: An Anglo- Celtic Cooperative Oncology Group study. J Clin Oncol 2005;23:2988-95.[LinkOut]
- Gradishar WJ, Wedam SB, Jahanzeb M, Erban J, Limentani SA, Tsai KT, et al. Neoadjuvant docetaxel followed by adjuvant doxorubicin and cyclophosphamide in patients with stage III breast cancer. Ann Oncol 2005;16:1297-304.[LinkOut]
- Untch M, Konecny G, Ditsch N. Dose dense sequential epirubicin paclitaxel as preoperative treatment of breast cancer: Results of a randomized AGO study. Proc Am Soc Clin Oncol 2002;21:a133.
- Jackisch C, von Minckwitz G, Raab G, Schuette M, Blohmer JU, Hilfrich J, et al. Primary endpoint analysis of the GEPAR-DUO study-preoperative chemotherapy (PCT) comparing dose-dense versus sequential adriamycin/docetaxel combination in operable breast cancer (T2-3, N0-2, M0) [abstract]. Breast Cancer Res Treat 2002;76:s50.
- Limentani SA, Brufsky AM, Erban J K, Jahanzeb M, Lewis D Blumenthal et al. Dose dense neoadjuvant treatment of women with breast cancer utilizing docetaxel and vinorelbine with growth factor support [abstract]. Proc Am Soc Clin Oncol 2003;22:33.
- Chen XS, Nie XQ, Chen CM, Wu JY, Wu J, Lu JS. Weekly paclitaxel plus carboplatin is an effective nonanthracycline-containing regimen as neoadjuvant chemotherapy for breast cancer. Ann Oncol 2010;21:961-7.[LinkOut]
- von Minckwitz G, Costa SD, Eiermann W, Blohmer JU, Tulusan AH, Jackisch C, et al. Maximized reduction of primary breast tumor size using preoperative chemotherapy with doxorubicin and docetaxel. J Clin Oncol 1999;17:1999-2005.[LinkOut]
- Miller KD, McCaskill-Stevens W, Sisk J, Loesch DM, Monaco F, Seshadri R, et al. Combination versus sequential doxorubicin and docetaxel as primary chemotherapy for breast cancer: A randomized pilot trial of the Hoosier Oncology Group. J Clin Oncol 1999;17:3033-7.[LinkOut]
- Malhotra V, Dorr VJ, Lyss AP, Anderson CM, Westgate S, Reynolds M, et al. Neoadjuvant and adjuvant chemotherapy (CT) with doxorubicin and docetaxel (DD) with surgery and radiation in locally advanced breast cancer [abstract]. Proc Am Soc Clin Oncol 2001;2:b6.
- Valero V, Esteva FJ, Sahin AA, Booser DJ, Strom EA, Esparza-Guerra LT, et al. Phase II trial of neoadjuvant chemotherapy with docetaxel and doxorubicin, surgery, adjuvant CMF, and radiotherapy ± tamoxifen in locally advanced breast cancer [abstract]. Breast Cancer Res Treat 2000;64:69.
- Bouzid K, Vinholes J, Salas F, Mickiewicz E, Valdivia S, Ostapenko V, et al. A Phase III trial of Taxotere and doxorubicin (AT) vs 5-fluorouracil, doxorubicin and cyclophosphamide (FAC) in patients with unresectable locally advanced breast cancer: An interim analysis [abstract]. Eur J Cancer 2001;37:s167.[LinkOut]
- von Minckwitz G, Costa SD, Raab G, Blohmer JU, Eidtmann H, Hilfrich J, et al. Dose-dense doxorubicin, docetaxel, and granulocyte colony-stimulating factor support with or without tamoxifen as preoperative therapy in patients with operable carcinoma of the breast: A randomized, controlled, open phase IIb study. J Clin Oncol 2001;19:3506-15.[LinkOut]
- Bines J, Vinholes J, Del Giglio A, Vasconcelos A, Cabral C, Gusmao C, et al. Neo-adjuvant chemotherapy with weekly docetaxel (taxotere) in poor prognosis locally-advanced breast cancer (LABC) [abstract]. Breast Cancer Res Treat 2002;76:s54.
- Therasse P, Mauriac L, Welnicka-Jaskiewicz M, Bruning P, Cufer T, Bonnefoi H, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin and fluorouracil with dose intensified epirubicin and cyclophosphamide+ filgrastim as neoadjuvant treatment in locally advanced breast cancer: An EORTC-NCIC-SAKK multicenter study. J Clin Oncol 2003;21:843-50.[LinkOut]
- Ellis GK, Green SJ, Russell CA, Royce ME, Perez EA, Livingston RB. SWOG 0012, a randomized phase III comparison of standard doxorubicin and cyclophosphamide followed by weekly paclitaxel versus weekly doxorubicin and daily oral cyclophosphamide plus G-CSF followed by weekly paclitaxel as Neoadjuvant therapy for inflammatory and locally advanced breast cancer. J Clin Oncol 2006;24:s12.
- Ardavanis A, Scorilas A, Tryfonopoulos D, Orphanos G, Missitzis I, Karamouzis M, et al. Multidisciplinary therapy of locally far-advanced or inflammatory breast cancer with fixed perioperative sequence of epirubicin, vinorelbine, and Fluorouracil chemotherapy, surgery, and radiotherapy: long-term results. Oncologist 2006;11:563-73.[LinkOut]
- Al-Tweigeri TA, Ajarim DS, Alsayed AA, Rahal MM, Alshabanah MO, Tulbah AM, et al. Prospective phase II study of neoadjuvant doxorubicin followed by cisplatin/docetaxel in locally advanced breast cancer. Med Oncol 2009:27:571-7.[LinkOut]
- Yerushalmi R, Hayes MM, Gelmon KA, Chia S, Bajdik C, Norris B, et al. A phase II trial of a neoadjuvant platinum regimen for locally advanced breast cancer: pathologic response, long-term follow-up, and correlation with biomarkers. Clin Breast Cancer 2009;9:166-72.[LinkOut]
- Jinno H, Sakata M, Hayashida T, Takahashi M, Mukai M, Ikeda T, et al. A phase II trial of capecitabine and docetaxel followed by 5-FU/epirubicin /cyclophosphamide (FEC) as preoperative treatment in women with stage II/III breast cancer. Ann Oncol 2010;21:1262-6.[LinkOut]
- Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145-53.[LinkOut]
- Robidoux A, Buzdar AU, Quinaux E, Jacobs S, Rastogi P, Fourchotte V, et al. A phase II neoadjuvant trial of sequential nanoparticle albumin-bound paclitaxel followed by 5-luorouracil/epirubicin/cyclophosphamide in locally advanced breast cancer. Clin Breast Cancer 2010;10:81-6.[LinkOut]
- Vujaskovic Z, Kim DW, Jones E, Lan L, McCall L, Dewhirst MW, et al. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperth 2010;26:514-21.[LinkOut]
- Yardley DA, Peacock NW, Dickson NR, White MB, Vazquez ER, Foust JT, et al. A phase II trial of neoadjuvant gemcitabine, epirubicin, and docetaxel as primary treatment of patients with locally advanced or inflammatory breast cancer. Clin Breast Cancer 2010;10:217-23.[LinkOut]
- Anton A, Ruiz A, Plazaola A, Calvo L, Seguí MA, Santaballa A, et al. Phase II clinical trial of liposomal-encapsulated doxorubicin citrate and docetaxel, associated with trastuzumab, as neoadjuvant treatment in stages II and IIIA HER2-overexpressing breast cancer patients. GEICAM 2003-03 study. Ann Oncol 2010 Jul 5. [Epub ahead of print][LinkOut]
- von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst 2008;100:552-62.[LinkOut]
- Steger GG, Galid A, Gnant M, Mlineritsch B, Lang A, Tausch C, et al. Pathologic complete response with six compared with three cycles of neoadjuvant epirubicin plus docetaxel and granulocyte colony-stimulating factor in operable breast cancer: results of ABCSG14. J Clin Oncol 2007;25:2012-8.[LinkOut]
- von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicincyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst 2008;100:542-51.[LinkOut]
- Verrill MW, Ashley SE, Walsh GA, Ellis P, Sacks N, Gui G, et al. Pathological complete response (pCR) in patients treated with neoadjuvant chemotherapy for operable breast cancer [abstract]. Breast Cancer Res Treat 1998;50:328.
- Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert F J, et al. Neoadjuvant chemotherapy in breast cancer: Enhanced response with docetaxel. J Clin Oncol 2002;20:1456-66.[LinkOut]
- Thomas E, Holmes FA, Smith TL, Buzdar AU, Frye DK, Fraschini G, et al. The use of alternate, non-cross-resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin-based regimen in women with operable breast cancer: Long-term results from a prospective randomized trial. J Clin Oncol 2004;22:2294-302.[LinkOut]
- De Lena M, Zucali R, Viganotti G, Valagussa P, Bonadonna G. Combined chemotherapy-radiotherapy approach in locally advanced (T3b-T4) breast cancer. Cancer Chemother Pharmacol 1978;1:53-9.[LinkOut]
- Hortobagyi GN, Blumenshein GR, Spanos W, Montague ED, Buzdar AU, Yap HY, et al. Multimodal Treatment of Locoregionally Advanced Breast Cancer. Cancer 1983;51:763-76.[LinkOut]
- Ragaz J, Baird R, Rebbeck P, Goldie J, Coldman A, Spinelli J, et al. Neoadjuvant (preoperative) chemotherapy for breast cancer. Cancer 1985;56:719-24.[LinkOut]
- Swain SM, Sorace RA, Bagley CS, Danforth DN, Bader J, Wesley MN, et al. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res 1987;47:3889-94.[LinkOut]
- Jacquillat C, Baillet F, Weil M, Auclerc G, Housset M, Auclerc M, et al. Results of a conservative treatment combining induction (neoadjuvant) and consolidation chemotherapy, hormonotherapy and external and interstitial irradiation in 98 patients with locally advanced breast cancer (IIIA-IIIB). Cancer 1988;61:1977-82.[LinkOut]
- Hortobagyi GN, Ames FC, Buzdar AU, Kau SW, McNeese MD, Paulus D, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery and radiation therapy. Cancer 1988;62:2507-16.[LinkOut]
- Perloff M, Lesnick GJ, Korzun A, Chu F, Holland JF. Combination chemotherapy with mastectomy or radiotherapy for stage III breast carcinoma: A Cancer and Leukemia Group B Study. J Clin Oncol 1988;6:261-9.[LinkOut]
- Valagussa P, Zambetti M, Bonadonna G, Zucali R, Mezzanotte G, Veronesi U. Prognostic factors in locally advanced noninflammatory breast cancer. Long-term results following primary chemotherapy. Breast Cancer Res Treat 1990;15:137-47.[LinkOut]
- Pierce LJ, Lippman M, Ben-Baruch N, Swain S, O'Shaughnessy J, Bader JL, et al. The effect of systemic therapy on local-regional control in locally advanced breast cancer. Int J Radiat Oncol Biol Phys 1992;23:949-60.[LinkOut]
- Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer 1994;73:362-9.[LinkOut]
- Smith IE, Walsh G, Jones A, Prendiville J, Johnston S, Gunterson B, et al. High complete remission rates with primary neoadjuvant infusional chemotherapy for large early breast cancer. J Clin Oncol 1995;13:424-9.[LinkOut]
- Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, et al. Combined-modality treatment of inflammatory breast carcinoma: Twenty years of experience at M.D. Anderson Cancer Center. Cancer Chemother Pharmacol 1997;40:321-9.[LinkOut]
- Touboul E, Lefranc JP, Blondon J, Buffat L, Deniaud E, Belkacemi Y, et al. Primary chemotherapy and preoperative irradiation for patients with stage II larger than 3 cm or locally advanced non-inflammatory breast cancer. Radiother Onocol 1997;42:219-29.[LinkOut]
- Brain E, Garrino C, Misset JL, Carbonero GI, ltzhaki M, Cvitkovic E, et al. Long-term prognostic and predictive factors in 107 stage 11/111 breast cancer patients treated with anthracycline-based neoadjuvant chemotherapy. British Journal of Cancer 1997;75:1360-7.[LinkOut]
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: Eight year experience of the Milan Cancer Institute. J Clin Oncol 1998;16:93-100.[LinkOut]
- Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicinbased neoadjuvant chemotherapy. J Clin Oncol 1999;17:460-9.[LinkOut]
- Ezzat AA, Ibrahim EM, Ajarim DS, Rahal MM, Raja MA, Stuart RK, et al. High complete response in locally advanced breast cancer using paclitaxel and cisplatin. Breast Cancer Res Treat 2000;62:237-44.[LinkOut]
- Cristofanilli M, Buzdar AU, Snelge N, Smith T, Wasaff B, Ibrahim N, et al. Paclitaxel in the multimodality treatment for inflammatory breast carcinoma. Cancer 2001;92:1775-82.[LinkOut]
- Van Praagh I, Amat S, Delva R, Leduc B, Mouret-Reynier MA, Lortholary A, et al. Induction chemotherapy in operable breast cancer by NET regimen: Multicentric phase II trial [abstract]. Proc Am Soc Clin Oncol 2001;20:a1889.
- Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol 2003;21:46-53.[LinkOut]
- Jahanzeb M, Brufsky A, Erban J, Lewis D, Limentani S. Dose-dense neoadjuvant treatment of women with breast cancer utilizing docetaxel, vinorelbine and trastuzumab with growth factor support [abstract]. J Clin Oncol 2005;23:s26.
- Raefsky E, Castillo R, Lahiry A, Thompson DS, Hanson S, Meng C, et al. Phase II study of neoadjuvant bevacizumab and trastuzumab administered with albumin-bound paclitaxel (NAB paclitaxel) and carboplatin in HER2 locally advanced breast cancer [abstract]. J Clin Oncol 2008;26:s37.
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer.J Clin Oncol 2005;23:3676-85.[LinkOut]
- Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377-84.[LinkOut]
- Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol 2010;28:2024-31.[LinkOut]
- Spector NL, Blackwell K, Hurley J, Harris JL, Lombardi D, Bacus S, et al. EGF103009, a phase II trial of lapatinib monotherapy in patients with relapsed/refractory inflammatorybreast cancer (IBC): Clinical activity and biologic predictors of response [abstract]. J Clin Oncol 2006;24:502.
- Cristofanilli M, Boussen H, Baselga J, Lluch A, Ben Ayed F, Friaha M, et al. A phase II combination study of lapatinib (TYKERB) and paclitaxel as neoadjuvant therapy in patients with newly diagnosed inflammatory breast cancer (IBC) [abstract]. San Antonio Breast Cancer Symposium;General Session. Breast Cancer Res Treat 2006;100:s5.
- Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 2003;30:s117-24.[LinkOut]
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76.[LinkOut]
- Miles D, Chan A, Romieu G, Dirix LY, Cortes J, Pivot X, et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO [abstract]. J Clin Oncol 2008;26:s1088.
- Robert NJ, Dieras V, Glaspy J, Brufsky A, Bondarenko I, Lipatov O, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) [abstract]. J Clin Oncol 2009;27:s42.
- Ardavanis A, Doufexis D, Kountourakis P, Malliou S, Karagiannis A, Kardara E, et al. Salvage therapy of pretreated advanced breast cancer with bevacizumab and paclitaxel every two weeks. A retrospective case review study. BMC Cancer 2009;9:338.[LinkOut]
- Kountourakis P, Doufexis D, Maliou S, Karagiannis A, Kardara E, Margari C, et al. Bevacizumab combined with two-weekly paclitaxel as first-line therapy for metastatic breast cancer. Anticancer Res 2010;30:2969-71.[LinkOut]
- Overmoyer P, Silverman P, Leeming R, Shenk R, Lyons J, Ziats N, et al. Phase II trial of neoadjuvant docetaxel with or without bevacizumab in patients with locally advanced breast cancer [abstract]. Breast cancer Res Treat 2004;88:s106.
- Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol 2006;24:769-77.[LinkOut]
- Hurvitz SA, Bosserman LD, Leland-Jones B, Thirwell M, Allison MK, Barstis J, et al. A multicenter, double-blind randomized phase II trial of neoadjuvant treatment with single-agent bevacizumab or placebo, followed by docetaxel, doxorubicin, and cyclophosphamide (TAC), with or without bevacizumab, in patients with stage II or stage III breast cancer [abstract]. J Clin Oncol 2008;26:562.
- Greil R, Moik M, Reitsamer R, Ressler S, Stoll M, Namberger K, et al. Neoadjuvant bevacizumab, docetaxel and capecitabine combination therapy for HER2/neu-negative invasive breast cancer: Efficacy and safety in a phase II pilot study. Eur J Surg Oncol 2009;35:1048-54.[LinkOut]
- Waintraub S E, Tuchman V. The role of preoperative neoadjuvant cytoreductive dose-dense bevacizumab plus docetaxel followed by bevacizumab-doxorubicin-cyclophosphamide regimen in locally advanced operable breast cancer [abstract]. J Clin Oncol 2009;27:s11524.
- Yardley DA, Raefsky E, Castillo R, Lahiry A, LoCicero R, Thompson D, et al. Results of a multicenter pilot study of weekly nab-paclitaxel, carboplatin with bevacizumab, and trastuzumab as neoadjuvant therapy in HER2+ locally advanced breast cancer with SPARC correlatives [abstract]. J Clin Oncol 2009;27:s15.
- von Minckwitz G, Eidtmann H, Loibl S, Blohmer JU, Costa SD, Fasching PA, et al. Integrating bevacizumab, everolimus, and lapatinib into current neoadjuvant chemotherapy regimen for primary breast cancer. Safety results of the GeparQuinto trial. Ann Oncol 2010 Jul 12. [Epub ahead of print][LinkOut]
- Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev 2008;34:453-75.[LinkOut]
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. New Engl J Med 2009;360:679-91.[LinkOut]
- Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM, et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer 2010;102:1099-105.[LinkOut]
- Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297-302.[LinkOut]
Cite this article as: Papademetriou K, Ardavanis A, Kountourakis P. Neoadjuvant therapy for locally advanced breast cancer: Focus on chemotherapy and biological targeted treatments’ armamentarium. J Thorac Dis 2010;2(3):160-170. doi: 10.3978/j.issn.2072-1439.2010.02.03.8
|




