Recommendations for the management of idiopathic pulmonary fibrosis in South Africa: a position statement of the South African Thoracic Society
Introduction
Idiopathic pulmonary fibrosis (IPF) is a very specific form of chronic, progressive fibroproliferative interstitial pneumonia of unknown aetiology limited to the lungs (1). The disease is characterised by progressive worsening of dyspnoea and lung function and is generally associated with a poor prognosis (1). Although the aetiology of IPF is unknown, the pathogenesis is believed to involve both genetic predisposition and environmental risk factors, including smoking, exposure to metal and wood dust, gastro-oesophageal reflux disease (GORD) and even some viral infections (2).
Several international evidence-based guidelines on the diagnosis and management of IPF and other interstitial lung diseases (ILDs) have been published and updated in the last decade, as the body of evidence for the use of some treatment modalities has grown, whilst others have been shown to be futile and even harmful to patients (1,3). The most notable recent developments have been the early use of antifibrotic drugs and anti-acid therapy. However, the routine use of high dose oral steroids, immunosuppressive drugs and anticoagulants has been abandoned (4).
Given the fact that IPF is often treated by non-pulmonary specialist physicians in South Africa (SA), and not all treatment modalities are currently available in the country, the South African Thoracic Society (SATS) has decided to review the most recent evidence for practical everyday local use.
The diagnosis of IPF
Defining IPF
It is crucial that IPF should be viewed as a very specific disease entity, and not as an “umbrella” term for all end-stage fibrotic lung diseases such as fibrotic non-specific interstitial pneumonia (NSIP) or chronic hypersensitivity pneumonitis. Furthermore, the classical histological features of usual interstitial pneumonia (UIP) are not specific for IPF, and can be observed in diseases such as rheumatoid lung disease and other ILD secondary to connective diseases, asbestosis, chronic hypersensitivity pneumonitis, drug-induced ILD and even sarcoidosis. The American Thoracic Society (ATS) and the European Respiratory Society (ERS) initially defined the diagnostic criteria in 2002, which were subsequently updated and refined in 2011 and 2015 (1,3,5). IPF is currently defined by a combination of clinical, radiological and histological features, although the latter is not always required to make a definitive diagnosis.
Clinical features, pulmonary function testing and auxiliary tests
IPF classically presents with insidious onset (at least 3 months) of unexplained dyspnoea on exertion in patients older than 50 years. On examination, patients frequently demonstrate digital clubbing and have bibasilar, late inspiratory (“Velcro” type) crackles. It is vital to exclude other known causes of ILD such as connective tissue diseases (including rheumatoid arthritis, systemic lupus erythematosus and scleroderma), drug and environmental exposures and other idiopathic interstitial pneumonias with a focussed history, examination and special investigations.
Pulmonary function tests typically show varying degrees of restrictive ventilatory impairment, with a low forced vital capacity (FVC) and a normal to high forced expiratory volume during the first second to FVC ratio (FEV1:FVC >70%). Peak expiratory flow (PEF) is often high early in the disease, and the diffusion capacity for carbon monoxide (DLCO) is usually significantly impaired. One exception to these classical lung function findings is that of combined pulmonary fibrosis and emphysema (CPFE), where lung volumes (FVC) are relatively preserved in the presence of a very low DLCO and the FEV1:FVC ratio may be normal or low. Dyspnoea in such patients is usually disproportional to the relatively preserved lung function (6). Patients with early IPF may still have a normal resting arterial oxygen saturation, but frequently desaturate on exercise.
Laboratory tests should focus on excluding connective tissue diseases, as pulmonary manifestations may precede the clinical rheumatological syndrome. Antinuclear factor (ANF) and rheumatoid factor (RF) should routinely be performed in practically all cases. Although low positive ANF and RF titres are not infrequently observed in IPF, high titres should be viewed as significant, and prompt investigation for a connective tissue disorder. Anti-cyclic citrullinated peptide, and tests for other rheumatological conditions (e.g., scleroderma, Sjögren’s syndrome and dermatomyositis) may additionally be considered. During the follow-up of patients without a diagnosed connective tissue disease ab initio, repeated clinical and serological examination may be performed to exclude the emergence of an underlying rheumatological condition.
Radiology
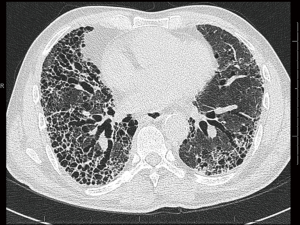
Routine chest radiographs may reveal decreased lung volumes, with prominent reticular interstitial markings, predominantly in the lung bases and lung periphery. High resolution computed tomography (HRCT) of the lungs is required in all cases of suspected IPF, and constitutes an essential component in the diagnosis of the disease. The presence of a definite UIP pattern on HRCT (Table 1), with reticulation, a basal predominance, subpleural honeycombing and traction bronchiectasis or bronchiolectasis (Figure 1), and the absence of features inconsistent with UIP (e.g., upper or middle zone predominance, extensive ground glass opacities, cysts, consolidation or air trapping) enable a multidisciplinary team to make a clinico-radiological diagnosis of IPF in a patient with no other known cause of UIP (1,3). The absence of honeycombing on HRCT, but fulfilling the remaining criteria, should be labelled as probable UIP, and requires histological confirmation.

Full table
Bronchoscopy
Bronchoscopy with transbronchial biopsy (TBB) and bronchoalveolar lavage (BAL) are not routine but are recommended if an alternate diagnosis is considered (e.g., hypersensitivity pneumonitis or sarcoidosis where a high lymphocyte count on BAL fluid would be inconsistent with IPF) (4).
Histology
The histopathological criteria for the diagnosis of IPF, as suggested in 2011, are summarised in Table 2 (1). Features that are against the diagnosis of UIP include marked interstitial inflammation (away from the areas of honeycombing), hyaline membranes or organising pneumonia (may be seen in acute exacerbations), granulomas, changes that are predominantly airway-centred and any features suggestive of an alternative diagnosis.

Full table
A practical diagnostic approach
In a patient who presents with classic clinical features, restrictive ventilatory impairment with impaired diffusion and a chest radiograph suggestive of IPF, an HRCT scan of the lungs is often all that is required to make a definitive diagnosis of IPF, provided it shows a definite UIP pattern (Table 1) and all other causes of a radiological UIP pattern are excluded. This evaluation should be performed by a physician/pulmonologist in consultation with a radiologist with experience in ILD interpretation.
Patients who present with atypical clinical features or an HRCT pattern that can only be classified as “possible” UIP, should be referred for a lung biopsy, which could be performed either with the aid of video-assisted thoracoscopic surgery (VATS) or a mini-thoracotomy. Biopsy sites should be discussed and identified prior to surgery and overtly fibrotic areas (“end-stage” lung) should be avoided. The diagnosis of IPF should be made by an experienced multidisciplinary team and based on the combination of clinical, radiological and histological findings. In some cases where patients are deemed unfit for surgical biopsy, a working diagnosis of IPF can be entertained based on the opinion of a multidisciplinary team.
Suggested management of patients with IPF
General approach and treatment options
IPF, in general, has a poor prognosis with a life expectancy of 2–5 years once diagnosed, although it is well known that significant variability in the disease progression can be observed. Some patients experience a very rapid decline in lung function, whereas other may have a more indolent course. Although clinicians often feel obliged to offer these patients medical management, most of the treatment options (e.g., high dose oral steroids, immunosuppressive drugs and anticoagulants) simply do not have evidence to support their use, and could potentially be harmful (3).
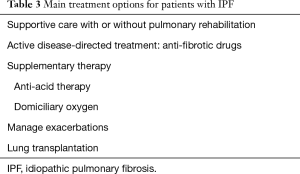
As with any chronic disease, a patient-centred approached should be followed, as the stage of the disease, degree of impairment, rate of disease progression, comorbid illnesses and patient preferences all impact on the long-term management. The main treatment options for patients with IPF are summarised in Table 3. More than one approach is often necessary and the various options are not mutually exclusive.
Full table
The medical treatment recommendations for IPF have changed rather dramatically over the past decade. Until 2008, most guidelines recommended triple therapy with prednisone, azathioprine and N-acetylcysteine (NAC).(7) This approach was abandoned after the PANTHER-IPF trial showed that this strategy was associated with increased hospitalisation and mortality rates (8). The 2011 ATS/ERS/JRS/ALAT evidence-based guidelines highlighted the overall lack of robust evidence at that time for any effective therapies, and suggested that patients should be offered best supportive care in conjunction with clinical trial recruitment where possible (1). The 2015 update of the ATS/ERS/JRS/ALAT guideline, however, conditionally recommends the use of pirfenidone and nintedanib for IPF, and consideration for anti-acid therapy (3).
Supportive care and follow up
Patents with relatively preserved lung function (FVC >80%), those with CPFE and patients with minimal symptoms can be judiciously followed up at 3 monthly intervals (4). A fall in FVC of >10% or DLCO of >15% suggest significant disease progression. Indeed, a decline in FVC of >10% over 6 months is associated with a 5-fold increased risk of mortality in the subsequent year (9). Patients with advanced disease or significant comorbidities that preclude the use of anti-fibrotic therapy on the other hand, should be offered symptomatic relief of breathlessness and supportive care, whilst ensuring any reversible causes of deterioration are quickly identified and managed (4). Referral to palliative care services should be arranged.
Supportive care should include influenza and pneumococcal vaccination. Additionally, the management of comorbidities is an essential component of clinical care (4). IPF patients are often elderly and other causes for breathlessness should be considered. Concomitant ischaemic heart disease and left ventricular failure are frequent causes for breathlessness. An echocardiogram is valuable in assessing left ventricular dysfunction and pulmonary hypertension, and smoking cessation should be offered to active smokers. Patients may have coexisting airways disease and inhaled bronchodilator therapy should be optimised.
Pulmonary rehabilitation (PR)
The role of PR is less well established in ILD than in chronic obstructive pulmonary disease (COPD). A Cochrane review concluded that PR was safe and resulted in improvements in functional exercise capacity, dyspnoea and quality of life immediately following the PR in IPF patients, but the quality of evidence was rated low to moderate (10). A recent randomised study, however, found that while IPF patients allocated to PR showed improvements in quality of life and physical activity, these changes were only present for the duration of the intervention and not 3 months thereafter (11). Further robust studies with long-term outcomes are needed, and there is currently insufficient evidence to support routine referral of IPF patients for PR (4).
Anti-fibrotic drugs: pirfenidone and nintedanib
Pirfenidone is an oral anti-fibrotic drug which inhibits pro-fibrotic cytokine cascades in vitro and fibroblast proliferation in animal models of lung fibrosis (12,13). The results of the first three randomised double-blind placebo-controlled trials were mixed (14-16). The ASCEND study used 2,403 mg pirfenidone/day (in 3 divided doses) vs. placebo in 555 patients (17). It met the primary end-point of significantly decreasing the rate of FVC decline at 1 year (193 mL less mean decline in absolute FVC: 235 vs. 428 mL; P=0.001) and a relative reduction of 48% in patients with ≥10% FVC fall (17). There was also a reduction in decline in the 6-minute walk test (6MWT) distance (decrease of ≥50 m, 26% vs. 36%) but there was no difference in respiratory symptoms. When pooled with the two previous CAPACITY trials with a total of 1,247 patients, there was a significant decrease in mortality (HR, 0.52; 95% CI, 0.31–0.87; P=0.01) (16). Both gastrointestinal (abdominal discomfort and loss of appetite) and skin-related (photosensitivity) adverse events were more common in the pirfenidone group but rarely led to treatment discontinuation. Thus pirfenidone is currently conditionally recommended therapy by both the ATS and ERS because of the reduction in FVC decline, lowering of mortality and absence of severe side-effects (3).
Nintedanib is a potent intracellular multi-target tyrosine kinase inhibitor which binds & blocks the receptors of vascular endothelial growth factor, platelet-derived growth factor and fibroblast growth factor (18). Three multicentre randomised double-blind placebo-controlled trials, the phase II TOMORROW and two phase III INPULSIS trials (19) investigated its efficacy and safety over 1 year in patients with IPF. In the INPULSIS trials the dose used was 150 mg twice daily but treatment interruption and/or dose reduction to 100 mg bd was allowed to ameliorate adverse events. Both trials met the primary end-point of significantly decreasing the decline in FVC at 1 year: 124 and 93 mL less in absolute FVC (115 vs. 239 mL/year and 114 vs. 207 mL/year; 95% CI, 77.7–172.8; P<0.0001) as well as a relative decrease of 52% and 45% in patients with ≥10% fall in FVC (pooled data RR, 0.63; 95% CI, 0.49–0.82, P=0.0007). Two key secondary end-points [improvement in quality-of-life as measured by the St. George’s Respiratory Questionnaire (SGRQ) plus time to first exacerbation] were met in INPULSIS-2 but not in INPULSIS-1. However, there was no difference in respiratory symptoms or frequency of acute exacerbations. There was also no significant lowering of mortality (HR, 0.7; 95% CI, 0.43–1.12, P=0.14) but both trials were underpowered for this end-point. Regarding adverse events, diarrhoea was common (62%) but 96% of patients tolerated the full dose. Pooled and meta-analyses were conducted for the TOMORROW trial and both INPULSIS trials to increase the patient numbers to 1,231 patients (20). The adjusted annual rate of decline in FVC was −112.4 mL/year with nintedanib and −223.3 mL/year with placebo (difference: 110.9 mL/year, 95% CI, 78.5–143.3; P<0.0001). Nintedanib significantly reduced the risk of on-treatment mortality vs. placebo (HR, 0.57; 95% CI, 0.34–0.97; P=0.0274). The proportion of patients who died during the on-treatment period was 3.5% in the nintedanib group vs. 6.7% in the placebo group. The hazard ratio for time to first acute exacerbation was 0.53 (95% CI, 0.34–0.83; P=0.0047). The adjusted mean change from baseline in SGRQ score at week 52 was 2.92 with nintedanib and 4.97 with placebo [difference: −2.05 (95% CI, −3.59 to −0.50); P=0.0095]. Thus nintedanib is also now conditionally recommended therapy by both the ATS and ERS because of its slowing of FVC decline and patient-centred outcomes, but patients need to be warned about the side-effect of diarrhoea (3).
Both pirfenidone and nintedanib modestly slow the rate of disease progression and have fairly manageable side effects but are very expensive and neither actually improves symptoms or quality of life. Of interest, the patients in the INPULSIS trials receiving nintedanib had less severe disease than those receiving pirfenidone in the ASCEND trial (mean predicted FVC of 80% vs. 68% of) as they did not exclude patients with a normal FVC. Several important questions remain: (I) is either agent effective in patients unlike those recruited (very early or more severe disease)? (II) What is the optimal duration of therapy? (III) How long will effectiveness last (>1 year)? (IV) Will side-effects remain acceptable? (V) Can we predict which patients will respond to one or both? (VI) Can they be used in combination? (VII) Are they effective in patients with co-morbidities (especially associated emphysema)? (VIII) Can they be used to treat acute exacerbations?
Neither drug is currently registered with the South African Medicines Control Council (MCC), but both are currently available under section 21. The SATS conditionally recommends, based on the current evidence and in accordance with the UK National Institute for Health and Care Excellence (NICE) guidelines as well that either drug should be offered to patients with IPF with an FVC 50–80% (21,22).Treatment should be discontinued if there is evidence of disease progression, which is currently defined as a decline in FVC of ≥10% within any 12-month period. The need for anti-fibrotic use with mild disease in order to prevent decline is unproven, and the drugs are considered less cost-effective in this situation.
Anti-oxidant therapy
Prior to 2012 the recommended therapy for IPF was the combination triple drug regimen of prednisone, azathioprine and the anti-oxidant NAC. This was in view of the 2005 IFIGENIA study which showed less decline in FVC and DLCO at 12 months when NAC was added to prednisone and azathioprine (23). However, the randomised placebo-controlled PANTHER trial showed that triple therapy was associated with increased all-cause mortality, all-cause hospitalisations and treatment-related serious adverse events and was thus stopped early (8). There was also no significant difference in FVC, DLCO or quality-of-life. It is therefore now strongly recommended that triple therapy should no longer be prescribed. As regards NAC monotherapy, two multicentre RCTs have shown no change in the FVC as well as no significant differences in the mortality rates, acute exacerbations, quality-of-life or adverse events (24,25). Thus NAC monotherapy is also not recommended.
Anti-acid therapy
Chronic microaspiration in patients with GORD may cause repetitive injury to the alveolar epithelium which in theory may play a part in the pathogenesis of IPF (4). It is known that IPF patients have significantly worse gastro-oesophageal reflux than controls, and pepsin originating from the gastrointestinal tract has been isolated in BAL fluid in patients with acute exacerbations of IPF (4,26). Interestingly, the use of anti-acid therapy has been associated with a slower decline in FVC over time and fewer acute exacerbations (27). Current guidelines recommend empirical treatment of gastro-oesophageal reflux in IPF patients, but good quality evidence is lacking (3,4). However, a recent post-hoc analysis of the CAPACITY 004, CAPACITY 006, and ASCEND data unfortunately failed to show improved outcomes in patients with IPF treated with antacids (28). In fact, there was a statically insignificant increased risk of infection in those with advanced disease, casting doubt on the value of offering anti-acid therapy to all patients with IPF, particularly patients with advanced disease (28).
Other medical interventions
The latest ATS/ERS/JRS/ALAT clinical practice guideline strongly recommends against the use of anticoagulation; combination prednisone, azathioprine and NAC; ambrisentan (a selective endothelin receptor) antagonist and imatinib (a tyrosine kinase inhibitor) (3). Moreover, the guideline conditionally discourages the use of dual endothelin receptor antagonists (macitentan and bosentan) and sildenafil (3). These recommendations are further endorsed by the SATS.
Domiciliary oxygen
Most guidelines, despite the lack of good quality evidence, suggest offering oxygen therapy as is prescribed in COPD (4). While the use of long-term oxygen therapy in COPD has been shown to have a mortality benefit, it is unclear whether these findings can be extrapolated to the IPF population (29). At least one randomised controlled trial has evaluated the effects of ambulatory oxygen on IPF patients without resting hypoxaemia but who desaturated during exercise. Oxygen levels were improved, but no significant differences in level of dyspnoea, leg fatigue or 6-minute walking distance were shown (30). Despite poor quality evidence, current guidelines strongly recommend domiciliary oxygen if hypoxia is present.
Acute exacerbations of IPF
There are no evidence based guidelines on the management of any aspect of acute exacerbations of IPF. The following is a resume of expert opinion on the subject (31). Acute exacerbations of IPF are defined as an idiopathic worsening of dyspnoea within the preceding 30 days with the appearance of new or worsening infiltrates on imaging, after exclusion of alternative causes. Respiratory infection must be excluded and, where appropriate, left ventricular dysfunction and pulmonary embolism. Expert opinion recommends management with corticosteroids unless contraindicated, although the optimal dose and duration are not known. Azathioprine or cyclophosphamide should not be prescribed and biologics, (e.g., rituximab) are not recommended. A broad spectrum antibiotic for community acquired or nosocomial infection, as appropriate, should be considered; while in general, anti-viral agents are not routinely recommended. Adjunctive therapy includes supplemental oxygen and psychological support. If the subject is on anti-fibrotic therapy this can be continued but should not be initiated at this stage. Continuous positive airway pressure (CPAP) can be useful, but intubation and mechanical ventilation are not considered standard of care.
Lung transplantation
The 2011 ATS guideline strongly recommends referral for lung transplantation in appropriate patients. IPF remains one of the most common indications for lung transplantation in the USA, with a 5-year survival of between 50% and 56% (32). In general IPF has a poor prognosis, with a median survival of 2 to 3 years from diagnosis, and approximately 25% of patients surviving >5 years (1). Because of this and due to the lack of locally available transplant centres and donor organs, patients considered for transplant should be referred early for a transplant eligibility assessment, and then monitored for timing of transplant listing (i.e., addition to waiting list). Experts recommend referral for assessment when FVC <80% predicted, DLCO <40% predicted, any dyspnoea due to lung disease or any oxygen requirement (33). Recent evidence suggest that bilateral lung transplantation is superior to single-lung transplantation in IPF (34,35). There are numerous contraindications to lung transplantation, and transplant assessment remains difficult even in experienced centres, but the greatest challenge in SA is access to centres experienced in transplantation and long-term post-transplant care.
Conclusions
It is pivotal to view IPF as a very specific form of a chronic, progressive fibroproliferative interstitial pneumonia of unknown aetiology. In a patient who presents with classic clinical features, restrictive ventilatory impairment with impaired diffusion and a HRCT scan of the lungs showing a UIP pattern, a definitive diagnosis of IPF can be made, provided all other causes of a radiological UIP pattern are excluded. Patients who present with atypical clinical features or an HRCT pattern that can only be classified as “possible” UIP, should be referred for a surgical lung biopsy.
Once the diagnosis of IPF is confirmed, a patient-centred approached should be followed, as the stage of the disease, degree of impairment, rate of disease progression, comorbid illnesses and patient preferences all impact on the long-term management. The SATS suggests that anti-fibrotic treatment should be offered to appropriate candidates (confirmed IPF with a FVC of 50–80%), but discontinued should there be evidence of disease progression (a decline in FVC of ≥10% within any 12-month period). The routine use of high dose oral steroids, immunosuppressive drugs and anticoagulants is not recommended whilst anti-acid therapy may be considered in patients without advanced disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: GM Ainslie and EM Irusen report an honorarium for having attended the Boehringer Ingelheim National Respiratory Advisory Board Meeting for Nintedanib. The other authors have no conflicts of interest to declare.
References
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Coghlan MA, Shifren A, Huang HJ, et al. Sequencing of idiopathic pulmonary fibrosis-related genes reveals independent single gene associations. BMJ Open Respir Res 2014;1:e000057. [Crossref] [PubMed]
- Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3-19. [Crossref] [PubMed]
- Fraser E, Hoyles RK. Therapeutic advances in idiopathic pulmonary fibrosis. Clin Med (Lond) 2016;16:42-51. [Crossref] [PubMed]
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Jankowich MD, Rounds SI. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest 2012;141:222-31. [Crossref] [PubMed]
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63 Suppl 5:v1-58. [Crossref] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968-77. [Crossref] [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:459-66. [Crossref] [PubMed]
- Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2014.CD006322. [PubMed]
- Jackson RM, Gómez-Marín OW, Ramos CF, et al. Exercise limitation in IPF patients: a randomized trial of pulmonary rehabilitation. Lung 2014;192:367-76. [Crossref] [PubMed]
- Nakazato H, Oku H, Yamane S, et al. A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level. Eur J Pharmacol 2002;446:177-85. [Crossref] [PubMed]
- Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 2008;590:400-8. [Crossref] [PubMed]
- Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040-7. [Crossref] [PubMed]
- Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821-9. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]
- Wollin L, Wex E, Pautsch A, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J 2015;45:1434-45. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS(®) trials. Respir Med 2016;113:74-9. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Pirfenidone for treating idiopathic pulmonary fibrosis. Available online: https://www.nice.org.uk/guidance/gid-tag504/documents/appraisal-consultation-document
- National Institute for Health and Care Excellence. Nintedanib for treating idiopathic pulmonary fibrosis. Available online: https://www.nice.org.uk/Guidance/TA379
- Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2229-42. [Crossref] [PubMed]
- Homma S, Azuma A, Taniguchi H, et al. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology 2012;17:467-77. [Crossref] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network, Martinez FJ, de Andrade JA, et al. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2093-101. [Crossref] [PubMed]
- Mertens V, Blondeau K, Vanaudenaerde B, et al. Gastric juice from patients "on" acid suppressive therapy can still provoke a significant inflammatory reaction by human bronchial epithelial cells. J Clin Gastroenterol 2010;44:e230-5. [PubMed]
- Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med 2013;1:369-76. [Crossref] [PubMed]
- Kreuter M, Wuyts W, Renzoni E, et al. Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis. Lancet Respir Med 2016;4:381-9. [Crossref] [PubMed]
- Cranston JM, Crockett AJ, Moss JR, et al. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005.CD001744. [PubMed]
- Nishiyama O, Miyajima H, Fukai Y, et al. Effect of ambulatory oxygen on exertional dyspnea in IPF patients without resting hypoxemia. Respir Med 2013;107:1241-6. [Crossref] [PubMed]
- Maher TM, Whyte MK, Hoyles RK, et al. Development of a Consensus Statement for the Definition, Diagnosis, and Treatment of Acute Exacerbations of Idiopathic Pulmonary Fibrosis Using the Delphi Technique. Adv Ther 2015;32:929-43. [Crossref] [PubMed]
- George TJ, Arnaoutakis GJ, Shah AS. Lung transplant in idiopathic pulmonary fibrosis. Arch Surg 2011;146:1204-9. [Crossref] [PubMed]
- Vandervest KM, Zamora MR. Recipient risk factors and lung transplant outcomes. Curr Opin Organ Transplant 2013;18:531-6. [Crossref] [PubMed]
- Schaffer JM, Singh SK, Reitz BA, et al. Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA 2015;313:936-48. [Crossref] [PubMed]
- Gulack BC, Ganapathi AM, Speicher PJ, et al. What Is the Optimal Transplant for Older Patients With Idiopathic Pulmonary Fibrosis? Ann Thorac Surg 2015;100:1826-33. [Crossref] [PubMed]


