Surgical technique for lung retransplantation in the mouse
Introduction
Physiologically relevant animal models are critical to advance our understanding of graft failure after lung transplantation in humans. Our laboratory has developed techniques for orthotopic vascularized lung transplantation in the mouse, which have allowed for the design of studies that examine mechanisms contributing to the high failure rate of pulmonary grafts (1,2). Retransplantation of various tissues has been utilized to study how graft-resident or -infiltrating cells regulate immune responses to specific grafts (3,4). We have recently developed and validated a method to retransplant mouse lungs, which we used to investigate the role of early alloimmune responses in pulmonary allograft rejection and acceptance (5). Here we provide a detailed technical description for left pulmonary retransplantation in the mouse utilizing cuff techniques that represents an addition to the available experimental models in lung transplantation.
Operation techniques
Left orthotopic vascularized lung transplants are performed in syngeneic or allogeneic strain combinations as previously described (1). Donor and recipient mice are anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection. At various time points ranging from 3 to 30 days after the initial transplant the lung grafts are harvested from these hosts. Animal procedures were approved by our institutional Animal Studies Committee.
Donor operation
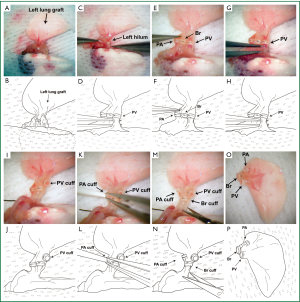
Anesthetized mice are intubated with a 20-gauge angiocatheter and connected to a ventilator with room air at a tidal volume of 0.5 mL and a respiratory rate of 110-120/min. 100 units of heparin are injected intravenously before the harvest. Left lung grafts are exposed through a median sternotomy and flushed with 3 mL of cold low-potassium dextran glucose (LPDG) solution through the main pulmonary artery after dividing the inferior and superior venae cavae. While harvesting the heart-lung block from the initial host, dissection needs to be performed carefully due to the formation of adhesions between the graft and the chest wall, which we typically encounter at the site of the original incision. With increased experience we found that in some cases, if adhesions are severe, it is preferable to leave a small piece of chest wall tissue on the surface of the graft to prevent injury to the graft, which could result in pneumothoraces. The heart-lung block is excised and the lung graft is prepared for retransplantation in a petri dish filled with ice cold LPDG solution (Figure 1A,E). The left hilum is dissected and the cuffs, which had been placed during the initial transplant, are exposed (Figure 1B,F). The 10-0 nylon suture ligatures, which had been used to secure the cuffs to the respective recipient vessels, are removed (Figure 1C,G). We recommend the use of 10-0 nylon to secure the cuffs to the recipient structures during the initial transplant because of the relative ease of the removal of monofilament as compared to braided suture material. Caution is required when removing these suture ligatures to avoid the accidental severing of the ligatures that have been used to secure the cuffs to the donor structures. The pulmonary vein and artery are cut proximal to the previously placed cuffs (24 G on pulmonary artery and 20 G on pulmonary vein). Subsequently the recipient vessels are dissected from the graft while maintaining the original cuffs (Figure 1D,H,I,M). Particularly when we perform the retransplant procedure at extended time periods after the initial engraftment (e.g., 30 days), it has been our experience that dense adhesions often prevent us from separating the recipient vessels from the donor vessels after release of the ligature. Under these circumstances, we divide the recipient vessel distal to the cuff, fold it over the cuff and secure it to the cuff with a 10-0 nylon tie. We then flush the lung graft with 1 mL of LPDG solution through the pulmonary artery (Figure 1J,N) and subsequently store the heart-lung block in LPDG solution at 4 °C. During this time period the graft bronchus remains cuffed (18 G) and attached to the recipient bronchus (Figure 1K,O). To prevent preservation solution from entering the airway during storage, the suture ligature that had been placed during the initial transplant procedure to secure the recipient to the donor bronchus is not released until the graft is retransplanted (Figure 1L,P).
Recipient operation
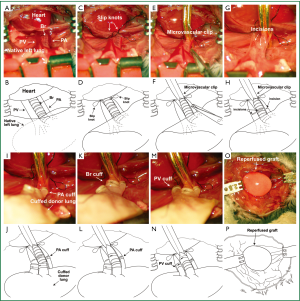
Secondary recipient mice are anesthetized, intubated orotracheally and placed in a right lateral decubitus position. A left thoracotomy is performed through the third intercostal space. A clamp is attached to the native left lung for lateral retraction and exposure of the hilum (Figure 2A,E). The hilum of the left lung is dissected and the pulmonary artery and vein are occluded temporarily with a slip knot (8-0 silk suture) (Figure 2B,F). The left main bronchus is occluded with a microvascular clip (Figure 2C,G). An incision is then made in each of these structures at the same level in preparation for the insertion of the cuffed donor structures (Figure 2D,H). At this point we separate the bronchus of the lung graft from the bronchus of the initial recipient (Figure 1K,O). As we have described for the vessels above, in case we encounter severe adhesions between the bronchus of the initial recipient and the cuff on the donor bronchus we cut the recipient bronchus distal to the cuff, fold the bronchus over the cuff and secure it with an additional 10-0 nylon tie. Cuffed donor pulmonary artery, vein and bronchus are inserted into the respective recipient structures and the cuffs are secured with 10-0 nylon suture ligatures (Figure 2I,J,K,M,N,O). Close attention needs to be paid to maintaining the proper orientation of the hilar structures during reimplantation of the graft to prevent their torsion, which can result in technical failure. This may necessitate lysis of adhesions within the graft hilum and dissection of the vessels and bronchus. The graft is reperfused and ventilated upon release of the ties and clip that had occluded the recipient structures (Figure 2L,P). The chest incision is closed in two layers with 6-0 nylon and the mouse is extubated.
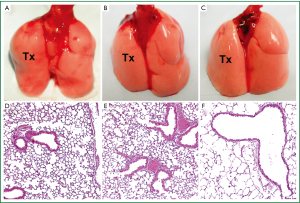
The total time of the retransplantation procedure is approximately 55 minutes, 20 minutes for the donor procedure and 35 minutes for the reimplantation. Warm ischemic times are comparable to primary lung transplants. To date, we have completed 144 retransplantation procedures with a technical success rate exceeding 95%. In syngeneic combinations, retransplanted grafts remain ventilated and free of inflammation for at least 3 months after retransplantation, similar to primary transplants (Figure 3).
Comments
Experiments using rodent models of graft retransplantation have yielded important insights in transplantation biology. For example, the immunogenic role of passenger leukocytes has been defined in organ parking experiments using re-transplanted rat kidneys (3). Retransplantation of lungs has also been reported in the rat model (6). Compared to rats, however, mice offer important advantages such as abundant transgenic strains and availability of reagents.
Cuff anastomotic techniques have been widely used for the transplantation of a variety of rodent organs. The use of cuff techniques that we have described for primary lung transplants has allowed us to retransplant mouse lungs. Employing this technique has recently allowed us to compare the role of early alloimmune responses in rejection and acceptance of lung and heart grafts (5). We found that, unlike the case for hearts, a short period of immunosuppression establishes regulatory pathways within lung grafts that allow them to survive for extended periods of time in nonimmunosuppressed allogeneic hosts.
The mouse lung retransplantation model represents a novel and powerful tool to examine how cells that reside in or infiltrate pulmonary grafts regulate immune responses. The importance of this model is highlighted by our recent demonstrations that the immune response to lung grafts differs from other organs (5,7).
Acknowledgements
We thank Arlene Ligori for medical illustration.
Funding sources: D.K. and A.E.G. are supported by a grant sponsored by The National Heart, Lung, and Blood Institute (1R01HL094601). D.K. and A.S.K. are supported by a grant sponsored by The National Heart, Lung, and Blood Institute (NIH R01 HL113931).
Disclosure: The authors declare no conflict of interest.
References
- Okazaki M, Krupnick AS, Kornfeld CG, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant 2007;7:1672-9. [PubMed]
- Li W, Sugimoto S, Lai J, et al. Orthotopic vascularized right lung transplantation in the mouse. J Thorac Cardiovasc Surg 2010;139:1637-43. [PubMed]
- Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med 1982;155:31-41. [PubMed]
- Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med 2002;195:1641-6. [PubMed]
- Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol 2012;5:544-54. [PubMed]
- Marui T, Iwata H, Shirahashi K, et al. Histologic damage of lung allografts according to magnitude of acute rejection in the re-isotransplant model. J Heart Lung Transplant 2008;27:642-8. [PubMed]
- Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol 2009;182:3969-73. [PubMed]


