Comparison of four DNA extraction methods for detecting Mycobacterium tuberculosis by real-time PCR and its clinical application in pulmonary tuberculosis
Introduction
Despite global efforts to control tuberculosis, it remains a major threat to public health. Most cases of tuberculosis occur in developing countries, especially those in southern and eastern Asia (1-4). In China, tuberculosis causes 205,000 deaths per year, making China second only to India in terms of tuberculosis mortality (5-8).
The most widely used routine methods for establishing a diagnosis of tuberculosis are direct smear for acid-fast bacilli and culture for Mycobacterium tuberculosis (MTB). The former method has the disadvantage of low sensitivity and the latter method has to be incubated for more than one month before a final diagnosis can be made.
One new approach for the rapid, safe and reproducible identification of MTB infection is PCR. The PCR method is attractive because of the possibility of directly detecting MTB in clinical specimens, including sputum and peripheral blood leukocytes (9-11). In this study, PCR method was used for the quantification of MTB DNA in plasma/serum samples. Previous research found that nonquantitative PCR of peripheral blood leukocytes was of little value for the specific diagnosis of pulmonary tuberculosis (12). Therefore, we used quantitative fluorescent PCR for the detection of MTB. Another important issue is the challenge for the earlier diagnosis of tuberculosis is the DNA extraction of MTB. In this study, four MTB DNA extraction methods were compared, including phenol-chloroform method, Qiagen kit, Omega kit and magnetic bead method. The best DNA extraction method was chosen for the quantitation of PCR method.
Materials and methods
Sample collection
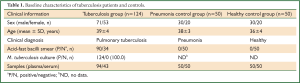
A total of 43 serum samples from 30 clinically diagnosed pulmonary tuberculosis patients and 94 plasma samples from 94 clinically diagnosed pulmonary tuberculosis patients were included in this study (Table 1). Serum samples before and after anti-tuberculosis chemotherapy were collected in 10 cases, while in other cases, only serum or plasma samples before chemotherapy were collected.
These samples were collected from Wuxi Hospital for Infectious Diseases in China from 2005 to 2010. Fifty plasma samples and 50 serum samples from pneumonia patients, 50 plasma samples and 50 serum samples from healthy volunteers were collected simultaneously (Table 1). These samples were stored at –70 °C.
MTB DNA and plasmid DNA preparation
MTB DNA was extracted by the phenol-chloroform method. We designed two complementary oligonucleotides: MA (5'-ACGGGAGCGGTTGGTGGTGGAAATCGTGCGTGACATTAAGA-3') and MB (5'-CTTAATGTCACGCACGATTTCCACCACCAACCGCTCCCGTA-3') using the Primer Express 2.0 software, which did not hybridize to any sequences in the human genome. The two complementary oligonucleotides were synthesized, then boiled for 5 min and cooled at room temperature. They were then confirmed by polyacrylamide gel electrophoresis. The synthesized DNA was recombined with PMD 18-T vector plasmid (Omega, American). Then, the plasmid was linearized with restriction enzyme HincII and purified through a QIAquick column (Qiagen, Germany).
Preparation of standard solutions of MTB DNA and plasmid DNA
MTB DNA and plasmid DNA was quantified using the GeneQuant II DNA calculator (Pharmacia). Ten-fold serial dilutions of the MTB DNA and plasmid DNA were prepared in 10 mmol/L Tris-HCl, pH 8.5, containing 10-104 copies of MTB DNA or plasmid DNA per microliter.
Four MTB DNA extraction methods
Blood from four healthy donors was collected in tubes containing EDTA. Plasma was pooled and stored at –80 °C. The linearized plasmid was used as a marker DNA. MTB DNA and plasmid DNA were added to plasma to obtain final concentrations of MTB DNA (6×102 copies/μL) and plasmid DNA (2×102 copies/μL). Then, four different DNA extraction methods were compared (Figure 1), including the phenol-chloroform method, the QIAamp DNA Mini Kit (Qiagen, German), the Omega DNA extraction kit (Omega, United States) and the magnetic bead kit (PerkinElmer, United States); the first DNA extraction method was performed according to the Molecular Cloning: A Laboratory Manual (Fourth Edition), the following three methods were performed according to the manufacturer’s instructions. Each test was performed with 10 replicates. After the final DNA extraction step, the DNA was dissolved in 40 μL of TE buffer. Once the most effective method had been selected, DNA was extracted from all of the clinical samples using this method.
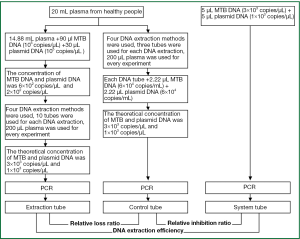
Primers and probes
The primers and probe used to identify MTB isolates were designed as previously reported, which targets the IS6110 gene (13). The sequences were as follows: forward primer F1: 5'-GGCTGTGGGTAGCAGACC-3', reverse primer R1: 5'-CGGGTCCAGATGGCTTGC-3', and the fluorescent probe P1: 5'-FAM-TGTCGACCTGGGCAGGGTTCG-TAMRA-3'. The expected amplification product size using primers F1 and R1 was 163 bp.
The primers and probe used to amplify plasmid DNA were designed using the Primer Express 2.0 software. The sequences were as follows: forward primer F2: 5'-AAACAGCTATGACCATGATTACGAA-3', reverse primer R2: 5'-CTTAATGTCACGCACGATTTCC-3', and the fluorescent probe P2: 5'-FAM-TGTCGACCTGGGCAGGGTTCG-ECLIPSE-3'. The expected amplification product size using primers F2 and R2 was 99 bp. All of the primers and probes were synthesized by Invitrogen.
Real-time PCR to amplify MTB DNA and plasmid DNA
Each 50-μL reaction contained 10 μL of 5× real time PCR buffer, 1.5 μL of 10 mM dNTPs, 0.7 μL of 250 mM MgCl2, 2 μL of each forward and reverse primer, 1 μL of fluorescent probe, 0.25 μL of Ex Taq HS (TaKaRa) and 5 μL of extracted DNA. The amplification conditions for tuberculosis DNA were as follows: 95 °C for 5 min, followed by 40 cycles of 94 °C for 30 s and 60 °C for 1 min. The amplification conditions for plasmid DNA were: 95 °C for 5 min, followed by 45 cycles of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 40 s. The DNA extraction efficiency, relative loss of marker DNA and DNA inhibition ratio were calculated according to a previously study (14).
PCR products of MTB DNA were revealed by horizontal electrophoresis on 2.0% agarose gel, and the PCR products were cloned by the TaKaRa TA cloning kit pMD-18 (TaKaRa, Dalian, China), transformed into E. coli DH5α and sequenced. The nucleotide sequences obtained were analyzed by using BLAST software on the National Center for Biotechnology website.
Statistical analysis
One-way ANOVA and subsequent Dunnett’s test or Bonferroni post-hoc test were used for statistical analysis of the data by State 7.0 software.
Results
Standard curves of MTB DNA and plasmid DNA
The regression equation for the MTB DNA amplification standard curve was Ct =–3.37 logC0 +34.56, r=0.9997. Whereas, the regression equation for the plasmid DNA amplification standard curve was Ct =–3.55 logC0 +39.31, r=0.9995. The linear ranges of the two amplifications were both from 1-104 copies/μL.
Comparison of the four MTB DNA extraction methods
Extraction efficiency
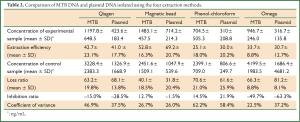
All experimental samples from the four methods had higher MTB and plasmid DNA concentrations than the control samples. Single-factor analysis of variance was used to compare the DNA concentration difference between the experimental and control samples. The results showed that the MTB DNA extracted by the magnetic bead method was of significantly higher concentration than that extracted using the phenol-chloroform method (P=0.003), and the plasmid DNA extracted by the magnetic bead method was of significantly higher concentration than that extracted using the Qiagen kit (P=0.003), the phenol-chloroform method (P<0.001) and the Omega kit (P<0.001), indicating that the magnetic bead method had the highest DNA extraction efficiency (Table 1).
Full Table
Relative loss ratio
Single-factor analysis of variance showed that the relative loss ratio of MTB DNA extracted by the magnetic bead method was significantly lower than that of the DNA extracted using the Qiagen kit (P=0.018) and the phenol-chloroform method (P=0.001), and the relative loss ratio of plasmid DNA extracted by the magnetic bead method was significantly lower than that extracted by the other three methods, including the Qiagen kit (P<0.001), the phenol-chloroform method (P=0.002) and the Omega kit (P<0.001). These results showed that the magnetic bead method had the lowest loss ratio and is therefore more suitable for plasma DNA extraction (Table 1).
Relative inhibition ratio and reproducibility
A significant difference in the MTB DNA concentration was detected between the experimental and control samples (P=0.040), however, no significant difference in the plasmid DNA concentration was observed between the experimental and control samples. To compare reproducibility, DNA was extracted 10 times and the results showed that the magnetic bead method and the Omega kit resulted in the lowest CV values.
MTB DNA detection results for the tuberculosis patients and healthy controls
Among the 124 clinically diagnosed pulmonary tuberculosis patients, the plasma or serum samples of 39 (31.5%) patients tested positive for MTB DNA. DNA sequencing of all the 39 positive PCR products confirmed that they were all MTB sequence by using BLAST software. Interestingly, 35.3% (12/34) of smear-negative tuberculosis cases were MTB DNA positive (Table 2, Figure 2). Serum samples before and after anti-tuberculosis chemotherapy were collected in 10 cases. Eight of these cases were MTB DNA negative before chemotherapy, and three of these cases gave a positive result after chemotherapy. The two cases that were MTB DNA positive before chemotherapy both gave a negative result after chemotherapy. All of the plasma and serum samples from the healthy controls were MTB DNA negative.
Full Table
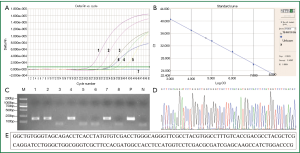
 Std, positive standard samples;
Std, positive standard samples;  unknown, test sample; C. Electrophoresis of fluorescent quantitative PCR amplification products: M, DNA marker (DL2000); 1-8, plasma/serum samples from tuberculosis patient 1-8; P, positive control; N, negative control; D. Sequence figure of MTB DNA amplification product; E. DNA sequence of MTB DNA amplification product.
unknown, test sample; C. Electrophoresis of fluorescent quantitative PCR amplification products: M, DNA marker (DL2000); 1-8, plasma/serum samples from tuberculosis patient 1-8; P, positive control; N, negative control; D. Sequence figure of MTB DNA amplification product; E. DNA sequence of MTB DNA amplification product.Discussion
M. tuberculosis, the causative agent of tuberculosis, is among the leading causes of death from infectious disease worldwide, especially in developing countries (8,15). Early identification of MTB is of great importance as it would help in the initiation of rapid appropriate treatment for patients (7,8,15-19). Real-time PCR is a methodology with high sensitivity that has the potential to detect MTB directly from clinical samples (20,21). DNA isolation is usually the first step towards the identification of MTB by real-time PCR assays. However, MTB DNA is difficult to isolate and there are a wide range of DNA isolation methods available which have been performed with varying levels of success. Therefore, in this study, we compared the efficiency of four DNA isolation methods and successfully detected MTB directly in plasma/serum specimens. This study reported the existence of circulating MTB DNA in the plasma/serum of tuberculosis patients, which providing a potential novel approach for the diagnosis of tuberculosis.
Circulating DNA, also known as cell-free DNA, refers to DNA that exists in the bloodstream but not in blood cells. It has long been known that circulating DNA is present in the plasma or serum of healthy and diseased individuals. Previous studies have reported that an increased amount of circulating DNA exists under many pathological conditions, such as autoimmune diseases, malignancy, prenatal diseases, trauma, organ dysfunction and stroke (22-29). However, whether circulating DNA exists in tuberculosis patients remains unknown. Furthermore, circulating DNA is an unspecific index which may increase during various diseases. Therefore, in this study, specific primers and probes were designed to detect MTB DNA in plasma and serum samples from tuberculosis patients.
A known amount of MTB DNA was added to the plasma samples from tuberculosis patients before DNA extraction. After DNA extraction using one of four different methods, including the phenol-chloroform method, the Qiagen kit, the Omega kit and the magnetic bead method, real-time PCR was performed to determine the extraction efficiency, the relative loss ratio and the inhibition ratio. For MTB DNA, the magnetic bead method had higher extraction efficiency than the phenol-chloroform method, and a lower relative loss ratio than the Qiagen kit and the phenol-chloroform method. For plasmid DNA, the magnetic bead method had the highest extraction efficiency and the lowest relative loss ratio compared with the other three DNA isolation methods. These results showed that the magnetic bead method was the most effective method for the isolation of MTB DNA from plasma samples.
Real-time PCR was also used for the accurate quantitative detection of MTB DNA in plasma/serum samples collected from patients with clinically diagnosed pulmonary tuberculosis and healthy controls. Of the tuberculosis patients, the plasma/serum samples of 31.5% (39/124) were positive for MTB DNA. Despite the detection rates being relatively low compared with sputum and other clinical samples (4,30-32), the plasma and serum samples were uniform and quantitative amplification was easy to perform. Interestingly, 35.3% (12/34) of smear-negative tuberculosis cases were MTB DNA positive. Tuberculosis remains a challenging diagnosis for both clinicians and microbiologists because of the low sensitivity of acid-fast bacilli smear method and the long testing cycle of MTB culture. However, if acid-fast staining is combined with the detection of MTB DNA in plasma/serum samples, the sensitivity of tuberculosis diagnosis may increase.
Serum samples before and after anti-tuberculosis chemotherapy were collected in 10 cases. Two cases which were MTB DNA positive before chemotherapy tested negative after chemotherapy, indicating a good prognosis for the patients. Of the other eight cases that were MTB DNA negative before chemotherapy, three tested positive after chemotherapy, indicating that this index may be useful in evaluating therapeutic effects.
Despite the limitations of this real-time PCR technique using plasma/serum samples, we believe that it is a useful tool for the diagnosis of tuberculosis, especially in smear-negative cases. It may also provide a good therapeutic effect index. Further studies are now required to improve the sensitivity of this technique.
Acknowledgements
This research was funded by a grant from the Important National Science & Technology Specific Projects of China (Approval No. 2013zx1000308-002), a grant from National Natural Science Foundation of China (Approval No. 81000754), a grant from the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (Approval No. XK201114), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure: The authors declare no conflict of interest.
References
- World Health Organization. Global tuberculosis control: surveillance, planning, financing (WHO/HTM/TB/2005.349). Geneva, Switzerland: World Health Organization, 2005.
- Sun YJ, Lim TK, Ong AK, et al. Tuberculosis associated with Mycobacterium tuberculosis Beijing and non-Beijing genotypes: a clinical and immunological comparison. BMC Infect Dis 2006;6:105. [PubMed]
- Ohkado A, Murase Y, Mori M, et al. Transmission of specific genotype streptomycin resistant strains of Mycobacterium tuberculosis in the Tokyo Metropolitan Area in Japan. BMC Infect Dis 2009;9:138. [PubMed]
- Frothingham R, Stout JE, Hamilton CD. Current issues in global tuberculosis control. Int J Infect Dis 2005;9:297-311. [PubMed]
- Smith KC, Armitige L, Wanger A. A review of tuberculosis: reflections on the past, present and future of a global epidemic disease. Expert Rev Anti Infect Ther 2003;1:483-91. [PubMed]
- Dou HY, Tseng FC, Lin CW, et al. Molecular epidemiology and evolutionary genetics of Mycobacterium tuberculosis in Taipei. BMC Infect Dis 2008;8:170. [PubMed]
- Zhang X, Guo J. Advances in the treatment of pulmonary tuberculosis. J Thorac Dis 2012;4:617-23. [PubMed]
- Yang XY, Zhang NM, Diao X, et al. Epidemiological analysis of pulmonary tuberculosis in Sichuan Province, China, 2000-2006. Int J Infect Dis 2008;12:534-41. [PubMed]
- Ieven M, Goossens H. Relevance of nucleic acid amplification techniques for diagnosis of respiratory tract infections in the clinical laboratory. Clin Microbiol Rev 1997;10:242-56. [PubMed]
- Soini H, Musser JM. Molecular diagnosis of mycobacteria. Clin Chem 2001;47:809-14. [PubMed]
- Nakajima C, Rahim Z, Fukushima Y, et al. Identification of Mycobacterium tuberculosis clinical isolates in Bangladesh by a species distinguishable multiplex PCR. BMC Infect Dis 2010;10:118. [PubMed]
- Rolfs A, Beige J, Finckh U, et al. Amplification of Mycobacterium tuberculosis from peripheral blood. J Clin Microbiol 1995;33:3312-4. [PubMed]
- Eisenach KD, Cave MD, Bates JH, et al. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis 1990;161:977-81. [PubMed]
- de Kok JB, Hendriks JC, van Solinge WW, et al. Use of real-time quantitative PCR to compare DNA isolation methods. Clin Chem 1998;44:2201-4. [PubMed]
- Yang XY, Li YP, Mei YW, et al. Time and spatial distribution of multidrug-resistant tuberculosis among Chinese people, 1981-2006: a systematic review. Int J Infect Dis 2010;14:e828-37. [PubMed]
- Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis 2010;14:131-40. [PubMed]
- Pai M, Minion J, Sohn H, et al. Novel and improved technologies for tuberculosis diagnosis: progress and challenges. Clin Chest Med 2009;30:701-16. [PubMed]
- Morgan M, Kalantri S, Flores L, et al. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis 2005;5:62. [PubMed]
- Kalantri Y, Hemvani N, Chitnis DS. Evaluation of real-time polymerase chain reaction, interferon-gamma, adenosine deaminase, and immunoglobulin A for the efficient diagnosis of pleural tuberculosis. Int J Infect Dis 2011;15:e226-31. [PubMed]
- Richardson ET, Samson D, Banaei N. Rapid Identification of Mycobacterium tuberculosis and nontuberculous mycobacteria by multiplex, real-time PCR. J Clin Microbiol 2009;47:1497-502. [PubMed]
- Krishnan MY, Radhakrishnan I, Joseph BV, et al. Combined use of Amplified Fragment Length Polymorphism and IS6110-RFLP in fingerprinting clinical isolates of Mycobacterium tuberculosis from Kerala, South India. BMC Infect Dis 2007;7:86. [PubMed]
- Ziegler A, Zangemeister-Wittke U, Stahel RA. Circulating DNA: a new diagnostic gold mine? Cancer Treat Rev 2002;28:255-71. [PubMed]
- Anker P, Stroun M. Circulating DNA in plasma or serum. Medicina (B Aires) 2000;60:699-702. [PubMed]
- Schwarzenbach H, Alix-Panabières C, Müller I, et al. Cell-free tumor DNA in blood plasma as a marker for circulating tumor cells in prostate cancer. Clin Cancer Res 2009;15:1032-8. [PubMed]
- Beck J, Urnovitz HB, Riggert J, et al. Profile of the circulating DNA in apparently healthy individuals. Clin Chem 2009;55:730-8. [PubMed]
- van der Vaart M, Pretorius PJ. The origin of circulating free DNA. Clin Chem 2007;53:2215. [PubMed]
- Hatta M, Sultan AR, Tandirogang N, et al. Detection and identification of mycobacteria in sputum from suspected tuberculosis patients. BMC Res Notes 2010;3:72. [PubMed]
- Shibuya Y, Shiozaki T, Hayashi M, et al. Efficacy of Amplicor PCR for the diagnosis of tuberculosis in respiratory specimens other than sputum. Tuber Lung Dis 2000;80:209-15. [PubMed]
- Lazar L, Rigó J Jr, Nagy B, et al. Relationship of circulating cell-free DNA levels to cell-free fetal DNA levels, clinical characteristics and laboratory parameters in preeclampsia. BMC Med Genet 2009;10:120. [PubMed]
- Negi SS, Anand R, Pasha ST, et al. Detection of M. tuberculosis in clinical samples of diversified nature by IS6110 based PCR. J Commun Dis 2006;38:325-32. [PubMed]
- Rebollo MJ, San Juan Garrido R, Folgueira D, et al. Blood and urine samples as useful sources for the direct detection of tuberculosis by polymerase chain reaction. Diagn Microbiol Infect Dis 2006;56:141-6. [PubMed]
- Cadmus SI, Jenkins AO, Godfroid J, et al. Mycobacterium tuberculosis and Mycobacterium africanum in stools from children attending an immunization clinic in Ibadan, Nigeria. Int J Infect Dis 2009;13:740-4. [PubMed]


