Statin treatment before percutaneous cononary intervention
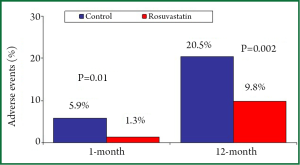
The prognostic benefits of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) in high-risk patients with stable coronary artery disease (CAD) and in those with acute coronary syndromes (ACS) are well established and their use is strongly recommended for secondary prevention (1-4). Two concepts arise from these studies about statin administration: earlier is better considering the time and higher is better considering the dose. In a cohort of 1,159 patients with acute myocardial infarction (MI), the statin treatment initiated within 48 hours after admission was associated with significantly lower major adverse cardiac events at one-year (17.8% vs. 24.6%; P=0.016) compared to statin treatment started after 48 hours; the early statin therapy was an independent predictor of one-year outcome [Odds Ratio (OR): 1.49; 95% Confidence Interval (CI): 1.0 to 2.21; P=0.045] (5). In patients with ACS, statin therapy may reduce one-month mortality (OR: 0.63; 95% CI: 0.41 to 0.99; P=0.047) when treatment is started very early, within 24 hours of hospitalization (6). The favourable effect of high-dose statin treatment is more evident in patients undergoing percutaneous coronary intervention (PCI). In the PROVE-IT TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) study, early intensive compared to moderate statin treatment, significantly reduced the composite end point of cardiovascular death, non fatal MI, recurrent ischemia or rehospitalisation for unstable angina in patients undergoing PCI [Hazar Ratio (HR): 0.73; 95% CI: 0.61 to 0.87; P=0.001] but not in those medically treated (HR: 0.84; 95% CI: 0.65 to 1.09; P=0.183), without a significant interaction between the dose of statin (intensive vs. moderate) and the treatment strategy (PCI vs. non PCI) (7). Few randomized studies have compared the prognostic impact of statin treatment when administered before PCI. However, available data suggest that pre-PCI statin administration may further improve prognosis. A recent meta-analysis of randomized trials that included stable CAD or ACS patients, showed a better 30-day outcome in the 1,649 patients randomized to short-term (from 12 hours to 2 weeks) high-dose statin compared to that of 1,649 patients randomized to low-dose or no statin therapy before PCI (8); in this meta-analysis, the rate of death, spontaneous non fatal MI or target vessel revascularization at 30-day was lower in patients pre-treated with statins (0.6% vs. 1.4%; OR: 44; 95% CI: 0.19 to 1.01; P=0.05). Moreover, non-ST elevation (NSTE) ACS statin-naïve patients (n=225) treated with high dose rosuvastatin (40 mg) 7-25 hours before PCI presented a better long-term outcome than patients (n=220) treated with rosuvastatin after intervention (Figure 1) (9). At 12 months, the composite primary end point of death, non-fatal MI, non-fatal stroke, or revascularization was significantly lower in the pre-PCI statin group (20.5% vs. 9.8%; P=0.002); similarly the risk of death and non-fatal MI at 12-month was significantly lower in patient who started rosuvastatin before PCI (HR: 3.71; 95% CI: 1.22 to 11.27; P=0.021) (9). Even a chronic statin therapy seems to improve long-term prognosis after PCI. Among 8,041 patients with stable CAD treated with PCI, 5,939 of these were on statin therapy (≥1 month) and 2,102 statin-naïve at the time of admission; statin therapy before PCI was associated with a significant reduction of one-year mortality (HR: 0.56; 95% CI: 0.42 to 0.75; P<0.001) with a benefit observed within the first month following PCI (10).
The beneficial clinical effects of statin pre-treatment may be related to their possible protective effects against both myocardial damage following PCI and kidney damage following contrast media administration. This review is focused on the relationship between the administration of statin therapy before PCI and the occurrence of periprocedural myocardial infarction (pMI) and contrast-induced acute kidney injury (CI-AKI).
Clinical evidence of statin benefits on periprocedural miocardial infarction prevention
Periprocedural myocardial infarction, as assessed by cardiac marker elevation, occurs in 5% to 40% of patients undergoing PCI, depending on the definition criteria used, antithrombotic therapies administered and clinical/angiographic risk profile of included patients (11-12). This complication may negatively impact the clinical outcome after intervention (13-15). Several observational studies showed that treatment with statins at the time of PCI in patients with stable CAD reduced the incidence of pMI (16-18).
The efficacy of short-term statin treatment before PCI has been first shown by the randomized ARMYDA (Atorvastatin for Reduction of Myocardial Damage during Angioplasty) study that enrolled statin-naïve patients undergoing elective PCI. In this study, a 7-day pre-treatment with atorvastatin (40 mg/day) was associated with 81% (5% vs. 18%; P=0.025) risk reduction of pMI defined by creatine kinase (CK)-MB elevation >2 times upper normal limit (UNL); this benefit was consistent even when different pMI definition criteria were used. Furthermore, at the multivariate analysis atorvastatin pre-treatment resulted independently associated with a lower rate of pMI (OR: 0.19; 95% CI: 0.05 to 0.57) (19). The benefit of short-term statin administration before PCI on pMI occurrence were confirmed in other studies including patients with stable CAD (20-22). The NAPLES II (Novel Approaches for Preventing or Limiting Events) trial was the first that showed that a single high-dose of atorvastatin (80 mg) given 24 h before elective PCI significantly reduced (9.5% vs. 15.8%; OR: 0.56; 95% CI: 0.45 to 0.89; P=0.014) the incidence of pMI (defined by CK-MB elevation >3 times UNL) in 668 stable CAD patients (23). Similar results on pMI occurrence (26.4% vs. 8.7%; P=0.003) were observed using high-dose rosuvastatin (40 mg) the day before PCI in 160 elective patients (24).
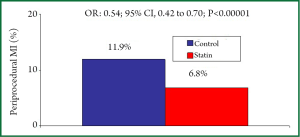
Other studies evaluated the role of statin pre-treatment in the setting of patients with ACS. In an observational study, Chang et al. demonstrated a lower incidence of pMI (2% vs. 19%; P=0.04) in patients with SCA already receiving statin treatment at the time of PCI compared to statin naïve patients (25). The ARMYDA-ACS (Atorvastatin for Reduction of Myocardial Damage during Angioplasty-Acute Coronary Syndromes) study was the first randomized, placebo-controlled trial that evaluated the effects of high-dose statin (atorvastatin 80 mg given 12 h before PCI with a further 40 mg dose 2 h before the procedure) administration before PCI in NSTE-ACS patients undergoing early (<48 h) PCI. In this study, the 30-day incidence of major adverse cardiac events (death, MI, target vessel revascularization) were significantly lower (5% vs. 17%; P=0.01) in the atorvastatin compared to the placebo group; the better 30-day outcome in the statin group was mostly driven by the reduction of pMI (5% vs. 15%; P=0.04) (26). These results were subsequently confirmed with a single high dose rosuvastatin (40 mg) given 16 h before PCI in patients with NSTE-ACS (27). In this randomized study the 30-day incidence of major cardiac events (death, non fatal MI, ischemic stroke, target vessel revascularization) was lower in the statin treatment arm (6.7% vs. 15.9%; P=0.002), and a pMI (defined by CK-MB elevation >2 times UNL) was detected in 5.8% of treated patients and in 11.4% of controls (P=0.035) (27). More recently, it has been shown that in ACS patients a very early (2-4 hours) rosuvastatin (20 mg) administration before PCI significantly reduce the pMI rate (8.1% vs. 22.2%; P28). In a meta-analysis of 13 statin randomized studies involving 3,341 patients, the incidence of pMI (defined by CK-MB elevation >3 times UNL) was 6.8% in the high-dose statin treated group and 11.9% in the the control group (OR: 0.54; 95% CI: 0.42 to 0.70; PFigure 2) (8). High-dose statin pretreatment was associated with a lower incidence of pMI in both stable CAD group (n=2,293) (7.5% vs. 13.2%; OR: 0.52; 95% CI: 0.41 to 0.66; Pvs. 9.0%; OR: 0.64; 95% CI: 0.40 to 1.02; P<0.06) without a significant interaction between treatment strategy and clinical presentation (P=0.43) (8). The role of high-dose statin administration on myocardial protection in patients with ST-elevation acute myocardial infarction (STEMI) undergoing primary PCI is still controversial. In the STATIN STEMI (Efficacy of High-Dose AtorvaSTATIN Loading Before Primary Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction) study, the pre-treatment with high dose atorvastatin (80 mg) improved coronary flow and microvascular coronary perfusion compared with low-dose atorvastatin (10 mg) (29). In the REPERATOR study (Prevention of REPERfusion Damage and Late Left Ventricular Remodeling With ATORvastatin Administered Before Reperfusion Therapy) the administration of high dose atorvastatina (80 mg) was not associated with improved cardiac function, microvascular perfusion or decreased myocardial infarct size compared to placebo (30).
Clinical evidence of statin benefits on contrast-induced acute kidney injury (CI-AKI) prevention
CI-AKI represents a possible complication of diagnostic and/or therapeutic procedures requiring administration of iodinated contrast media and comports prolonged hospitalization, increased costs, and increased short-and-long term morbidity and mortality (31). The prognostic impact of CI-AKI depends on the degree of kidney injury and the persistence of renal function deterioration (32-33). The incidence of CI-AKI varies widely depending on the patient cohorts evaluated, definition criteria used and preventive strategies adopted (34). We have recently reported the prognostic importance of both transient and persistent renal damage after CI-AKI development (33). In particular, transient and persistent renal damage were independent predictors of long-term clinical outcome increasing 1.6-fold and 2.5-fold, respectively, the risk of mortality, dialysis or major cardiovascular events at 5-year follow-up (33).
Observational and randomized studies suggest that statins therapy started before contrast medium administration might reduce CI-AKI incidence (35-49).
A review of a large database showed that statin therapy is associated with a lower incidence of CI-AKI after PCI (35) and similar results were retrospectively observed after contrast-medium exposure in patients with chronic renal impairment (36). In a prospective follow-up study on 430 patients (260 statin-treated and 174 statin naïve) followed for 4 years, those receiving statins before PCI had a significantly lower incidence of CI-AKI (3% vs. 27%; P37). Renal protection was observed even in patients more prone to develop postprocedural renal failure, such as the older and diabetic patients and this favourable effect resulted in a significantly shorter length of hospital stay. Patients with severely impaired baseline renal function (creatinine clearance 37). The majority of the data regarding the role of statins on CI-AKI prevention in elective patients, derived from non-randomized studies (36-41). A meta-analysis of these non-randomized studies showed a significant reduction of CI-AKI in statin treated patients compared to controls (OR: 0.60; 95% CI: 0.36 to 1.00; P=0.05) (46).
So far, only three randomized studies were performed in elective patients. The results of these studies did not show significant benefits with high-dose simvastatin or atorvastatin administered shortly before contrast agent injection (42-44). A favorable non significant trend toward beneficial protective effect of high dose atorvastatin (80 mg twice daily before PCI followed by 80 mg/daily for two days after the procedure) against CI-AKI (defined as increase of serum creatinine of at least 0.5 mg/dL or 25% within 2 days) occurrence (3% vs. 10%) was observed in a small randomized study involving 130 patients without baseline renal impairment (creatinine clearance >70 mL/min) (42). On the other hand, two randomized studies performed in patients with baseline renal dysfunction did not show any acute reno-protective effect of statins after contrast medium administration. In the PROMISS (Prevention of Radiocontrast-Medium-induced nephropathy using short term high-dose simvastatina) study, short-term high-dose simvastatin (40 mg twice daily, 48 h before procedure) did not prevent renal function deterioration after contrast medium administration but the CI-AKI rate (defined as increase of serum creatinine of at least 0.5 mg/dL or 25% within 2 days) in this study was very low in both the treated group (n=118, CI-AKI =2.5%) and controls (n=118; CI-AKI =3.4%) (43). Toso et al., performed a randomized placebo controlled trial in 304 patients with baseline renal dysfunction (creatinine clearance <60 mL/min) and higher clinical risk profile of CI-AKI than patients in the PROMISS study because the subjects were older, with lower baseline creatinine clearance and ejection fraction, and treated with PCI in a higher percentage of cases (44). Also in this study high-dose atorvastatin (80 mg/die 48 h before and after constrast exposure) did not reduce the CI-AKI rate (defined as increase of serum creatinine of at least 0.5 mg/dL or 25% within 5 days) which occurred in 10% of the treated patients and in 11% of controls (44). A meta-analysis of these three randomized studies showed a non significant 26% reduction of CI-AKI in the statin treated patients compared to controls (OR: 0.76; 95% CI: 0.41 to 1.41; P=0.39) (46).
More recently, Quintavalle et al., in 410 stable patients with chronic kidney disease enrolled in the NAPLES II trial, observed that high-dose atorvastatin (80 mg) administered the day before contrast medium significantly reduced the CI-AKI rate (defined as an increase in serum Cystatin-C concentration of 10% above the baseline value at 24 hours from contrast administration) in patients with creatinine clearance vs. 17.8%; OR: 0.22; 95% CI: 0.01 to 0.69; P45). In the additional in vitro experiment, they showed that the atorvastatin pre-treatment of non human tubular epithelial cells and human embryonic proximal tubule cells prevented renal cell apoptosis induced by contrast medium and this effect seemed to be mediated by a reduction in the stress kinases activation and restoration of the cell survival signals (45).
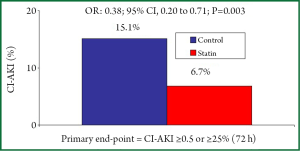
Patients with ACS have a three times higher risk of developing CI-AKI, an often serious complication that produces persistent worsening of renal function in 30% of cases (50-51). Early administration of high-dose lipophilic statins before contrast medium exposure resulted in a significant lower rate of CI-AKI in three randomized studies (47-49). Xinwei et al., compared 228 statin-naïve patients with ACS treated with high- (80 mg) or low-dose (20 mg) simvastatin before PCI. All patients had ejection fraction >40%, only 5% presented with chronic kidney disease and the majority (43%) had unstable angina as clinical presentation (NSTE-ACS =28%, STEMI =29%). In this study, medium-term (7.1±1.6 days) high-dose simvastatin was more effective than low-dose simvastatin in protecting kidney function after PCI, with a lower (66% relative reduction) CI-AKI rate (defined as increase of serum creatinine of at least 0.5 mg/dL or 25% within 2 days) (5.3% vs. 15.7%; P47). The ARMYDA-CIN study, randomized 241 statin-naïve ACS patients (unstable angina 66%; NSTE-ACS =34%) with ejection fraction >30%, to high-dose atorvastatin (80 mg given 12 h before PCI with a further 40 mg dose 2 h before the procedure) or placebo before PCI; after the procedure, all patients received atorvastatin 40 mg/day irrespective of the initial randomization assignment. In this study, high-dose atorvastatin significantly reduced the CI-AKI rate (defined as increase of serum creatinine of at least 0.5 mg/dL or 25% within 2 days) by 62% (5% vs. 13.2%; P<0.05) and the atorvastatin pre-treatment resulted an independent predictive factor against CI-AKI (OR: 0.34; 95% CI: 0.12 to 0.97; P<0.043) (48). The results of the PRATO-ACS (Protective effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome) trial have been recently presented at the Late Breaking Clinical Trial session of the American College of Cardiology Congress 2013 in San Francisco. In this study 504 statin-naïve ACS patients (NSTE-ACS =92%, unstable angina =8%) scheduled for early invasive strategy were randomly assigned to receive rosuvastatin (40 mg on-admission following by 20 mg/day; n=252) or no statin treatment (n=252). The incidence of CI-AKI (defined as an increase in creatinine ≥0.5 mg/dL or ≥25% above baseline within 72 hours) was significantly lower in the statin group than in controls (6.7% vs. 15.1%; OR: 0.38; 95% CI: 0.20 to 0.71; P=0.003) (Figure 3). The benefits against CI-AKI were consistent even applying different CI-AKI definition criteria and in the high risk categories such as patients with creatinine clearance vs. 20.9%; OR: 0.35; 95% CI: 0.25 to 0.81; P<0.014), those with ejection fraction ≤45% (10.7% vs. 24.7%; OR: 0.37; 95% CI: 0.16 to 0.85; P<0.020) and those undergoing PCI (7.0% vs. 14%; OR: 0.46; 95% CI: 0.22 to 0.95; P<0.036). The 30-day incidence of adverse cardiovascular and renal events (death, dialysis, MI, stroke and persistent renal damage) was significantly lower in the statin group (3.6% vs. 7.9%; P=0.036) (49).
Thus, at present, in stable patients undergoing elective diagnostic or therapeutic procedures, the evidence for the use of statins prior to contrast medium exposure in order to prevent CI-AKI development are still debated and this is mainly due to the low number of available randomized studies that included small cohorts of patients (42-44). Moreover, in some studies the statin treatment has been associated with the N-acetylcysteine (42,44,45) and in this case the possibility of an interaction or a synergistic effect between the two agents cannot be ruled out. In addition, a very short duration of statin treatment after contrast exposure (43,44) could limit its nephroprotective effects given the local persistence of contrast in the kidneys for more than 48 hours (52).
Instead, the results of the studies exploring the nephroprotective role of statins in ACS patients are more defined with the exception of STEMI patients undergoing primary PCI who are not still evaluated in randomized studies (53-54).
Mechanism of statin benefit on pMI and CI-AKI following PCI
Several studies have demonstrated an association between elevated pre-and-post PCI inflammatory status and adverse events following revascularization (55-57). In particular, high pre-procedural levels of high sensitive C-reactive protein (hs-CRP) were associated with a higher risk of adverse events after coronary stent implantation (18,56). However, patients who presented reduced hs-CRP levels after statin therapy showed a better clinical outcome compared to patients with persistently high hs-CRP levels, regardless of their LDL cholesterol values (57). Moreover, the preprocedural hs-CRP level resulted an independent predictor of adverse outcome only in patients who did not receive statin therapy before PCI (18).
Experimental and clinical evidence suggest that the beneficial properties of statins may be attributed to a variety of non-lipid lowering pleiotropic effects. By inhibiting HMG-CoA reductase, statins could also inhibit the synthesis of important isoprenoid intermediates, such as farnesyl-pyrophosphate and geranyl-geranyl-pyrophosphate that lie downstream from the mevalonic acid. These intermediates serve for the post-translational modification of intracellular proteins such as nuclear lamins, Ras, Rho, Rac and Rap (58). The inhibition of these intracellular isoprenoid dependent proteins may contribute to some of the biological effects of statins, including improved endothelial function, increased nitric oxide bioavailability, antioxidant effects, anti-inflammatory and immuno-modulatory properties, and antithrombotic activity, which may occur rapidly and at very low drug concentrations (59-60). The vascular endothelium that is functionally and structurally altered by atherosclerosis represents the main target of the action of statins. The administration of high doses of statins leads to coronary endothelial protection through a rapid improvement in vasoreactivity and inhibition of inflammatory responses (61-63).
A significant increase in inflammatory markers has been documented after PCI but this increase was significantly lower in patients treated with high doses of statins. This effect on inflammatory response is a class effect being evident using simvastatin (47), atorvastatin (48) or rosuvastatin (9,27). In particular, in the study of Xinwei et al., levels of hs-CRP protein, P-selectin, and intercellular adhesion molecule-1 significantly increased 24 hours after PCI compared to the pre-PCI values (P<0.001) but their increase was lower in patients treated with high-dose as compared to low-dose simvastatin (P<0.001) (47).
Of note, the attenuation of vascular injury and inflammation obtained with statin treatment leads to a reduced release of myocardial damage biomarkers especially in patients with higher pre-PCI inflammatory status (8,23). In the NAPLES study, a significant interaction was found between treatment strategy and baseline hs-CRP levels (F=8.12; P<0.004 by the ANCOVA model): atorvastatin therapy was significantly associated with cardio-protective effects in the subgroup of patients with higher baseline hs-CRP levels (4.6% vs. 16.5%; P<0.016) but not in those with normal hs-CRP (11.1% vs. 15%; P=0.18) (23). The meta-analysis of statin randomized studies confirmed these results showing that the protective effect of statin before PCI was more evident in patients with high inflammatory status (8). In the subgroup of 1,861 patients with normal baseline hs-CRP levels, the incidence of pMI was 7.8% in the high-dose statin group and 10.9% in the control group (OR: 0.69; 95% CI: 0.50 to 0.95; P<0.021), whereas in those 734 patients with elevated baseline hs-CRP levels it was 4.3 and 12.3%, respectively (OR: 0.32; 95% CI: 0.18 to 0.58; P<0.001) (8).
It is interesting that pleiotropic effects elicited by statins could be attenuated over time in patients on chronic therapy (64) and “recaptured” with a loading high dose (65). In the ARMYDA (Atorvastatin for Reduction of Myocardial Damage during Angioplasty)-RECAPTURE, 383 statin-treated patients (>30 days) with both stable (53%) and unstable (47%) coronary disease undergoing PCI were randomized to receive placebo or high loading dose of atorvastatin (80 mg 12 h before PCI and a further 40 mg dose 2 h before PCI). Similar to that observed in statin-naïve patients, the reload of high-dose atorvastatin in patients chronically treated with statins reduced the 30-day adverse clinical events (3.7 vs. 9.4%; P=0.037) and this benefit was mostly driven by the reduction of pMI (3.7% vs. 8.9%) (65). Even in this study, the pre-PCI inflammatory status influenced the efficacy of high-dose statin treatment. Infact, the multivariate analysis identified atorvastatin reload as an independent predictor of better 30-day outcome (OR: 0.50; 95% CI: 0.20 to 0.80; P=0.039), but the impact on 30-day outcome was different according to clinical presentation: stable CAD patients did not benefit from atorvastatin reload (4 vs. 4.9%; OR: 0.74; 95% CI: 0.20 to 2.90; P=0.70) as compared to ACS patients (3.3 vs. 14.8%; OR: 0.18; 95% CI: 0.10 to 0.83; P=0.027) (65).
Although the pathogenesis of CI-AKI is not completely understood, multiple mechanisms may be involved and in particular inflammatory processes play a crucial role (31). Patients who developed CI-AKI have post-PCI hs-CPR levels significantly higher than patients without CI-AKI (48). It is possible to speculate that the reduction of systemic inflammation exerted by statins may lead to favorable effects on CI-AKI development. Moreover, the protection against CI-AKI may also be related to a local anti-inflammatory effect of statins in the kidney. Several studies have shown that the inhibition of the enzyme HMG-CoA reductase reduces the receptor-mediated endocytosis in proximal tubule cells and consequently the tubule reabsorption of proteins (66-68). The process of protein reabsorption is locally pro-inflammatory and contributes to tubulointerstitial fibrosis (67). Therefore, statins therapy may be renoprotective by blocking protein reabsorbition in the tubule cells.
Iodinated contrast medium is water soluble, freely filtered by the glomerulus, and avidly taken up by renal tubule cells in the loop of Henle; when it is retained in the tubule cells and peritubular space for several days it can exert a direct acute tubule cells toxicity (52). Thus, reducing protein trafficking across the proximal tubule cells, statin might attenuate the inflammation, endothelial dysfunction and tubulointerstitial disease complicating iodinated contrast medium administration.
Conclusions
Available data suggest that pre-treatment with statins before PCI is associated with a better short- and long-term prognosis. Current guidelines recommend the use of high doses of statins before PCI to reduce the risk of periprocedural myocardial infarction in statin naïve patients (class IIa A) and in those on chronic statin therapy (class IIa B) (4). Actually, the favourable clinical effect exerted by statins may be related not only to the reduction of periprocedural myocardial damage but also to the reduction of acute renal injury caused by contrast medium administration. Both the myocardial and kidney protection might result from an attenuation of inflammatory processes carried out by the pleiotropic effects of statins.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35. [PubMed]
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504. [PubMed]
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711-8. [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44-122. [PubMed]
- Kim MC, Ahn Y, Cho KH, et al. Early statin therapy within 48 hours decreased one-year major adverse cardiac events in patients with acute myocardial infarction. Int Heart J 2011;52:1-6. [PubMed]
- Angeli F, Reboldi G, Mazzotta G, et al. Statins in acute coronary syndrome: very early initiation and benefits. Ther Adv Cardiovasc Dis 2012;6:163-74. [PubMed]
- Gibson CM, Pride YB, Hochberg CP, et al. Effect of intensive statin therapy on clinical outcomes among patients undergoing percutaneous coronary intervention for acute coronary syndrome. PCI-PROVE IT: A PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) Substudy. J Am Coll Cardiol 2009;54:2290-5. [PubMed]
- Patti G, Cannon CP, Murphy SA, et al. Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: a collaborative patient-level meta-analysis of 13 randomized studies. Circulation 2011;123:1622-32. [PubMed]
- Yun KH, Oh SK, Rhee SJ, et al. 12-month follow-up results of high dose rosuvastatin loading before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol 2011;146:68-72. [PubMed]
- Ndrepepa G, King L, Cassese S, et al. Prehospital statin therapy and one-year mortality in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Eur J Intern Med 2013;24:145-50. [PubMed]
- Klein LW, Kramer BL, Howard E, et al. Incidence and clinical significance of transient creatine kinase elevations and the diagnosis of non-Q wave myocardial infarction associated with coronary angioplasty. J Am Coll Cardiol 1991;17:621-6. [PubMed]
- Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. N Engl J Med 2011;364:453-64. [PubMed]
- Nallamothu BK, Bates ER. Periprocedural myocardial infarction and mortality: causality versus association. J Am Coll Cardiol 2003;42:1412-4. [PubMed]
- Cavallini C, Savonitto S, Violini R, et al. Impact of the elevation of biochemical markers of myocardial damage on long-term mortality after percutaneous coronary intervention: results of the CK-MB and PCI study. Eur Heart J 2005;26:1494-8. [PubMed]
- Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J 2005;26:2493-519. [PubMed]
- Herrmann J, Lerman A, Baumgart D, et al. Preprocedural statin medication reduces the extent of periprocedural non-Q-wave myocardial infarction. Circulation 2002;106:2180-3. [PubMed]
- Chan AW, Bhatt DL, Chew DP, et al. Early and sustained survival benefit associated with statin therapy at the time of percutaneous coronary intervention. Circulation 2002;105:691-6. [PubMed]
- Chan AW, Bhatt DL, Chew DP, et al. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation 2003;107:1750-6. [PubMed]
- Pasceri V, Patti G, Nusca A, et al. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2004;110:674-8. [PubMed]
- Briguori C, Colombo A, Airoldi F, et al. Statin administration before percutaneous coronary intervention: impact on periprocedural myocardial infarction. Eur Heart J 2004;25:1822-8. [PubMed]
- Bozbas H, Yildirir A, Mermer S, et al. Does pravastatin therapy affect cardiac enzyme levels after percutaneous coronary intervention? Adv Ther 2007;24:493-504. [PubMed]
- Kinoshita M, Matsumura S, Sueyoshi K, et al. Randomized trial of statin administration for myocardial injury: is intensive lipid-lowering more beneficial than moderate lipid-lowering before percutaneous coronary intervention? Circ J 2007;71:1225-8. [PubMed]
- Briguori C, Visconti G, Focaccio A, et al. Novel approaches for preventing or limiting events (Naples) II trial: impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. J Am Coll Cardiol 2009;54:2157-63. [PubMed]
- Sardella G, Conti G, Donahue M, et al. Rosuvastatin pretreatment in patients undergoing elective PCI to reduce the incidence of myocardial periprocedural necrosis: the ROMA trial. Catheter Cardiovasc Interv 2013;81:E36-43. [PubMed]
- Chang SM, Yazbek N, Lakkis NM. Use of statins prior to percutaneous coronary intervention reduces myonecrosis and improves clinical outcome. Catheter Cardiovasc Interv 2004;62:193-7. [PubMed]
- Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol 2007;49:1272-8. [PubMed]
- Yun KH, Jeong MH, Oh SK, et al. The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol 2009;137:246-51. [PubMed]
- Wang Z, Dai H, Xing M, et al. Effect of a Single High Loading Dose of Rosuvastatin on Percutaneous Coronary Intervention for Acute Coronary Syndromes. J Cardiovasc Pharmacol Ther 2013. [Epub ahead of print]. [PubMed]
- Kim JS, Kim J, Choi D, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv 2010;3:332-9. [PubMed]
- Post S, Post MC, van den Branden BJ, et al. Early statin treatment prior to primary PCI for acute myocardial infarction: REPERATOR, a randomized placebo-controlled pilot trial. Catheter Cardiovasc Interv 2012;80:756-65. [PubMed]
- McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008;51:1419-28. [PubMed]
- James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 2011;123:409-16. [PubMed]
- Maioli M, Toso A, Leoncini M, et al. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation 2012;125:3099-107. [PubMed]
- McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol 2006;98:5K-13K. [PubMed]
- Khanal S, Attallah N, Smith DE, et al. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med 2005;118:843-9. [PubMed]
- Attallah N, Yassine L, Musial J, et al. The potential role of statins in contrast nephropathy. Clin Nephrol 2004;62:273-8. [PubMed]
- Patti G, Nusca A, Chello M, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol 2008;101:279-85. [PubMed]
- Bouzas-Mosquera A, Vázquez-Rodríguez JM, Calviño-Santos R, et al. Statin therapy and contrast-induced nephropathy after primary angioplasty. Int J Cardiol 2009;134:430-1. [PubMed]
- Kandula P, Shah R, Singh N, et al. Statins for prevention of contrast-induced nephropathy in patients undergoing non-emergent percutaneous coronary intervention. Nephrology (Carlton) 2010;15:165-70. [PubMed]
- Yoshida S, Kamihata H, Nakamura S, et al. Prevention of contrast-induced nephropathy by chronic pravastatin treatment in patients with cardiovascular disease and renal insufficiency. J Cardiol 2009;54:192-8. [PubMed]
- Zhao JL, Yang YJ, Zhang YH, et al. Effect of statins on contrast-induced nephropathy in patients with acute myocardial infarction treated with primary angioplasty. Int J Cardiol 2008;126:435-6. [PubMed]
- Ozhan H, Erden I, Ordu S, et al. Efficacy of short-term high-dose atorvastatin for prevention of contrast-induced nephropathy in patients undergoing coronary angiography. Angiology 2010;61:711-4. [PubMed]
- Jo SH, Koo BK, Park JS, et al. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial--a randomized controlled study. Am Heart J 2008;155:499.e1-8.
- Toso A, Maioli M, Leoncini M, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol 2010;105:288-92. [PubMed]
- Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation 2012;126:3008-16. [PubMed]
- Pappy R, Stavrakis S, Hennebry TA, et al. Effect of statin therapy on contrast-induced nephropathy after coronary angiography: a meta-analysis. Int J Cardiol 2011;151:348-53. [PubMed]
- Xinwei J, Xianghua F, Jing Z, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol 2009;104:519-24. [PubMed]
- Patti G, Ricottini E, Nusca A, et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty--contrast-induced nephropathy] trial. Am J Cardiol 2011;108:1-7. [PubMed]
- Toso A. Protective effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome (PRATO-ACS). ClinicalTrial.gov Identifier: NCT01185938.
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393-9. [PubMed]
- Marenzi G, Ferrari C, Marana I, et al. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovasc Interv 2012;5:90-7. [PubMed]
- McCullough PA, Khambatta S, Jazrawi A. Minimizing the renal toxicity of iodinated contrast. Circulation 2011;124:1210-1. [PubMed]
- Zhao JL, Yang YJ, Zhang YH, et al. Effect of statins on contrast-induced nephropathy in patients with acute myocardial infarction treated with primary angioplasty. Int J Cardiol 2008;126:435-6. [PubMed]
- Bouzas-Mosquera A, Vázquez-Rodríguez JM, Calviño-Santos R, et al. Statin therapy and contrast-induced nephropathy after primary angioplasty. Int J Cardiol 2009;134:430-1. [PubMed]
- Dibra A, Mehilli J, Braun S, et al. Inflammatory response after intervention assessed by serial C-reactive protein measurements correlates with restenosis in patients treated with coronary stenting. Am Heart J 2005;150:344-50. [PubMed]
- Walter DH, Fichtlscherer S, Sellwig M, et al. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol 2001;37:839-46. [PubMed]
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20-8. [PubMed]
- Zhou Q, Liao JK. Pleiotropic effects of statins. -Basic research and clinical perspectives-. Circ J 2010;74:818-26. [PubMed]
- Liao JK. Clinical implications for statin pleiotropy. Curr Opin Lipidol 2005;16:624-9. [PubMed]
- Corsini A, Ferri N, Cortellaro M. Are pleiotropic effects of statins real? Vasc Health Risk Manag 2007;3:611-3. [PubMed]
- Anderson TJ, Meredith IT, Yeung AC, et al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med 1995;332:488-93. [PubMed]
- Crisby M, Nordin-Fredriksson G, Shah PK, et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 2001;103:926-33. [PubMed]
- Patti G, Chello M, Pasceri V, et al. Protection from procedural myocardial injury by atorvastatin is associated with lower levels of adhesion molecules after percutaneous coronary intervention: results from the ARMYDA-CAMs (Atorvastatin for Reduction of MYocardial Damage during Angioplasty-Cell Adhesion Molecules) substudy. J Am Coll Cardiol 2006;48:1560-6. [PubMed]
- Mensah K, Mocanu MM, Yellon DM. Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: a potential role for phosphatase and tensin homolog deleted on chromosome ten? J Am Coll Cardiol 2005;45:1287-91. [PubMed]
- Di Sciascio G, Patti G, Pasceri V, et al. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol 2009;54:558-65. [PubMed]
- Abbate M, Zoja C, Corna D, et al. In progressive nephropathies, overload of tubular cells with filtered proteins translates glomerular permeability dysfunction into cellular signals of interstitial inflammation. J Am Soc Nephrol 1998;9:1213-24. [PubMed]
- Agarwal R. Statin induced proteinuria: renal injury or renoprotection? J Am Soc Nephrol 2004;15:2502-3. [PubMed]
- Harper CR, Jacobson TA. Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol 2008;51:2375-84. [PubMed]


