The diagnostic yield and complications of open lung biopsies in kidney transplant patients with pulmonary disease
Introduction
Kidney transplantation is the treatment of choice for patients with end-stage renal disease. Kidney transplantation is associated with a substantial reduction in the risk of mortality and cardiovascular events, as well as a clinically relevant improvement in the quality of life compared to dialysis (1). However, the maintenance of immunosuppressive therapy leads to an increased risk of infection, cancer and drug toxicities (2-8).
The lungs are frequently involved in a variety of complications following kidney transplantation. Pulmonary disease can occur in up to 36% of these patients and contributes significantly to the increased morbidity and mortality (9). Infectious diseases are the most common causes of pulmonary complications. Pulmonary infections are the main cause of acute respiratory failure (7) and severe sepsis (10-12), while community-acquired pneumonia is the leading cause of admission to the intensive care unit (ICU) (13). The incidence of neoplasia and drug-related pulmonary toxicity is lower (7,14,15).
Lung biopsies with histopathology and microbiology analyses are often needed for a specific diagnosis in transplant recipients (6). An open lung biopsy (OLB) is considered the best diagnostic method for patients with diffuse lung disease (16-19). In immunosuppressed patients, specific diagnoses can be obtained in up to 80% of cases (20). An OLB is probably more relevant when clinical judgment, laboratory tests and high quality imaging studies do not allow for an accurate diagnosis in patients who failed to improve on broad-spectrum antibiotics (21). However, there are safety concerns related to the procedure (22,23). As some of the available evidence suggests that establishing a diagnosis not necessarily change management or affects clinical outcomes (16,24), the potential benefits of this invasive procedure need to be carefully assessed.
The purpose of this study was to assess the role of an OLB in determining the specific diagnosis in kidney transplant patients with pulmonary disease of unknown cause and the complications related to this procedure.
Methods
We conducted a single center, retrospective study in a 16-bed ICU of a kidney transplant center in Brazil (25). From April 2010 to April 2014, we consecutively assessed all adult kidney transplant patients (>18 years) admitted to the ICU who underwent an OLB to investigate lung disease. We included patients with acute respiratory failure as well as those who were admitted immediately after an elective OLB. There were no exclusion criteria. In the case of multiple procedures on the same patient, only the first procedure was included. The indication for OLB is not written in our center’s protocol. However, the procedure is usually indicated for all patients with deteriorating clinical or radiologic conditions despite previous investigation and empiric antibiotic treatment [electronic supplementary material (ESM)]. The local ethics committee (Comitê de Ética em Pesquisa – Universidade Federal de São Paulo) approved the study under the number 125.965 and waived the need for informed consent.
Data from electronic medical records were retrospectively collected by one of the authors (DYVT). We recorded the following data: patient demographics, transplant characteristics, the time frame from symptom onset to the date of the OLB, the simplified acute physiology score 3 (SAPS 3) at ICU admission, the Sequential Organ Failure Assessment (SOFA) score in the 24 hours before the OLB, the main radiological characteristics of the pulmonary lesions on the following day (16,26), and related procedures, such as bronchial lavage or trans-bronchial biopsies. We also analyzed the data regarding complications related to the biopsy, pathologic and microbiologic results, therapeutic changes after the OLB results (the addition or withdrawal of drugs), and ICU and hospital mortality rates.
Experienced thoracic surgeons (NT and EPOP) carried out all of the OLBs. The surgical approach was a lateral muscle-sparing thoracotomy performed in the operating room under general anesthesia. The site of the lung biopsy was previously determined by a review of computed tomography (CT) studies. In general, two specimens were obtained from the most affected regions. Each tissue specimen was divided into two portions and was sent for microbiologic and histopathological analysis.
We defined Group 1 as those patients who were admitted electively to the ICU in the immediate postoperative period of the OLB. Group 2 was defined as those patients who had an indication of an OLB during their ICU stay because of acute respiratory failure. The diagnosis was considered specific when the findings where characteristic enough to establish an etiology and direct therapy was initiated by the attending physician (e.g., infections, neoplasms, or autoimmune diseases). Organizing pneumonia was classified as specific (27). We define changes in therapy as the addition or discontinuation of at least one therapeutic strategy in the current treatment based on the OLB findings.
Complications included arrhythmias with hemodynamic instability or cardiac arrest during the procedure; the need for or increase in the noradrenaline dose (>0.1 µg/kg/min) within the first six hours after the procedure; acute myocardial infarction, stroke or major bleeding requiring transfusions or surgical approach within the first 72 hours after the procedure; reoperations for surgical complications; need for new chest drainage; prolonged air leak (maintenance of the chest tube for seven or more days); surgical wound complications (infection and hematoma) or other late complications.
Statistics
Descriptive statistics were used to characterize the study population. Continuous variables were described as the mean ± standard deviation (SD) or the median (25−75% quartiles) and compared using Student’s t-test or the Mann-Whitney U test. These variables were tested for normality using the Kolmogorov-Smirnov test. Categorical variables were described as the number (%) and were compared using the Chi-square test. To assess if the OLB could worsen organ dysfunctions, we analyzed the SOFA score variation, and the preoperative score was compared with the postoperative score using the Wilcoxon test or the paired t-test.
We also performed a multivariate logistic regression using the forward likelihood ratio (LR) method to determine which independent variables were predictors of hospital mortality. The variables with a P value of <0.10 in the univariate analysis were selected. The model calibration was assessed using the Hosmer-Lemeshow test, which was considered appropriate if P>0.10. We did not include the variables with missing data greater than 10%. We used SPSS 19.0 for Windows (SPSS, Chicago, IL, USA) for statistical analysis.
Results
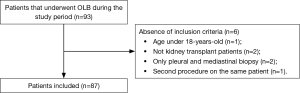
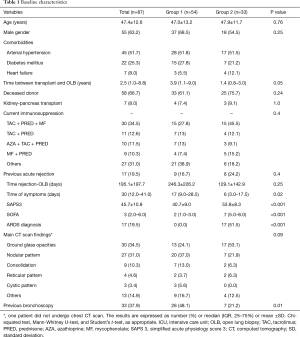
Ninety-three patients underwent OLB during the study period. However, we analyzed the data from 87 patients (Figure 1). Demographic and clinical characteristics of the study population are shown in Table 1. Patients in Group 2 were more severely ill and had a shorter duration of symptoms.
Full table
All patients received or were receiving at least one antibiotic therapy regimen before an OLB and showed no clinical and radiological improvement. Overall, a diagnosis was determined from an OLB in 74 (85.1%) patients (Table 2). In three cases, the results showed two different diseases: two patients with tuberculosis and histoplasmosis and one patient with tuberculosis and cytomegalovirus (CMV) pneumonia.

Full table
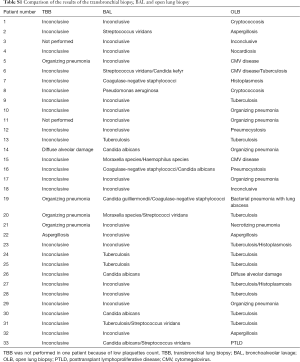
Fifty-four patients (62%) were not submitted to a diagnostic flexible bronchoscopy prior to OLB. By analysis of medical records, we were not able to identify the reasons why OLB was the first invasive diagnostic method option in most patients. Among the 33 (38%) patients who underwent a previous bronchoscopy, the transbronchial lung biopsy (TBB) provided specific diagnosis in five patients (four with organizing pneumonia and one with invasive pulmonary aspergillosis), but the OLB was indicated because the patients had an inadequate clinical response (Table S1). In the four patients with organizing pneumonia, the new histopathologic exam suggested the diagnosis of CMV, necrotizing pneumonia, tuberculosis, and bacterial pneumonia. The patient with aspergillosis had the same diagnosis after the OLB. Four patients had a positive result in bronchoalveolar lavage (BAL) culture for Mycobacterium tuberculosis. However, the definitive results were delayed, given the slow growth of Mycobacterium tuberculosis. A comparison of the results of the TBB, BAL and OLB are provided in the ESM.
Full table
The rate of specific diagnoses was similar in Group 1 (n=48, 89%) and Group 2 (n=26, 79%), P=0.62. Among the Group 2 patients, pneumocystis was the most common diagnosis (n=11, 33.3%), while in Group 1 the most common diagnosis was tuberculosis (n=15, 27.8%).
There was no association between the specific diagnosis and mortality, with 18 (24.3%) deaths in the group with specific diagnoses and four (30.7%) deaths in those without a diagnosis (P=0.2). In 46 patients (53%), the treatment was changed, a new treatment was introduced in 35 (40.2%) patients and medications were withdrawn in 17 (19.5%) patients.
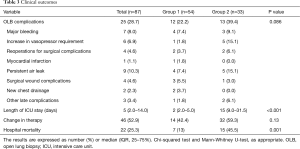
Twenty-five (28.7%) patients had complications related to the OLB (Table 3). The presence of complications was associated with a higher mortality rate (44% vs. 17.7%, P=0.01). There was a higher number of complications in patients from Group 2, although there was no statistical significance (39.4% vs. 22.2%, P=0.09). Overall, the SOFA score increased after the procedure (4.0±3.1 vs. 5.3±3.4, P<0.001). The changes in the SOFA score were similar in both groups [Group 1: 1.0 (0.0–2.0); Group 2: 0.0 (0.0–1.5); P=0.29].
Full table
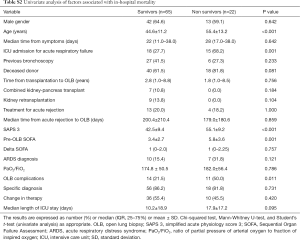
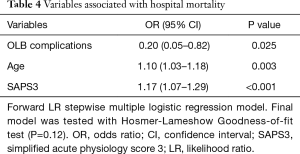
The hospital mortality rate was 25.2%, and the rate was higher in Group 2 than in Group 1 (45.5% vs. 13%, P=0.001). One patient in Group 2 died in the first 24 hours after the procedure from sepsis-related multiple organ dysfunctions. The univariate analysis of risk factors for hospital mortality is shown in Table S2 in the ESM. Age, the SAPS3 score and complications related to the procedure were independent predictors of all-cause hospital mortality in the multivariable regression model (Table 4).
Full table
Full table
Discussion
In our single center and observational study, the OLB was successful in reaching specific diagnoses in kidney transplant patients with undiagnosed diffuse pulmonary infiltrates and who failed to respond to empiric antibiotic treatment. However, OLB complications were common and were associated with increased hospital mortality.
The diagnostic yield of OLB, which does not specifically address solid organ transplant patients, ranges between 34% and 100% (16,17,19,28-41). In solid organ transplant patients, Defranchi et al. reported that 29% had a change in clinical management as a result of the OLB findings (16). In a heterogeneous group of immunocompromised patients with acute respiratory distress syndrome of unknown etiology, the biopsy resulted in major changes in management in 89% of the patients (39). Our study, which was conducted exclusively in kidney transplant recipients, showed that OLB resulted in a specific diagnosis in 85% of the patients and led to changes in treatment in 53% of the patients. The variability of OLB in impacting clinical management among these studies may be secondary to a number of methodological factors, including the use of a convenience sampling, the differences in study populations, the criteria used to define specific diagnoses and changes in treatment. We should also consider differences in the settings and time-dependent changes in management, as some of these studies are outdated.
The high rate of specific diagnoses and the changes in treatment with an OLB can be partially attributed to infection as a major cause of the pulmonary disease in our study. This finding was expected, as infection is highly prevalent among immunosuppressed patients who have undergone solid-organ transplantation (16). Others factors that could explain the high rates of infection are regional epidemiological characteristics, such as endemic infectious diseases (42), and the lack of laboratory assays utilizing molecular techniques or antigen detection that could facilitate early diagnosis and avoid invasive procedures (6). Another reason for our high rate of specific diagnosis is the low percentage of patients submitted to flexible bronchoscopy with BAL and TBB prior to OLB. In clinical practice the number of patients submitted to flexible bronchoscopy prior OLB may be less than expected (43-46). Some of the potential explanations include lesions localization, conditions that could increase the risk of complications such as high levels of positive end-expiratory pressure, risk of bleeding, and pulmonary hypertension, and the general believe in the low diagnostic yield of the procedure (43).
Actually, the overall diagnostic yield of flexible bronchoscopy is variable, ranging from 30% to 73% in solid organ transplant recipients; it is generally highest for pneumonia caused by Pneumocystis and lowest for mycobacterial or fungal etiologies (47). However, recent studies suggest that flexible bronchoscopy has a high diagnostic yield in immunocompromised patients with pulmonary infiltrates (48-50). Some studies suggest a higher diagnostic yield with OLB compared to flexible bronchoscopy, most of them old studies including patients previously submitted to TBB (36,51-53). In AIDS patients, OLB had a higher chance of reaching a specific diagnosis and changing therapy, as compared with the flexible bronchoscopy (40). Moreover, the diagnostic yield of flexible bronchoscopy may be lowest in critically ill patients, in exams performed late in the course of pulmonary infiltrates assessment and after initiation of antimicrobial therapy (47). There is a general belief that OLB should be the preferred method for lung biopsy in kidney transplant recipients with severe respiratory impairment (54). Actually, the likelihood that a procedure provides the information needed to establish a diagnosis probably depends on the diagnostic workup used by pathologists and microbiologists rather than on the methods used to obtain the tissue sample (54).
We need to take into account the risk associated with OLB in this population and balance both the potential benefits and risks. In our study, 28.7% of the patients had OLB complications, a finding that was similar to a solid organ transplant patient study (28%) (16). A recent meta-analysis demonstrated that the complication rate for an OLB in ARDS patients was 22% (55). Clearly, the different definitions of complications and population heterogeneity preclude direct comparisons among these studies. OLB complications occurred more frequently in patients with acute respiratory failure in our study, although there was no statistical significance. A similar finding was reported in patients with diffuse pulmonary infiltrates (31). Previous reports describing the high mortality in patients who underwent an OLB has raised safety concerns regarding the procedure (22). We found that OLB complications were independently associated with intra-hospital mortality. Our results suggest that this is a procedure that should be considered only after all other diagnostic attempts have failed. However, it is important to state that our study design does not allow for the assessment of causality.
Although we included only kidney transplant recipients, our study has two different group of patients. The group of patients admitted to the ICU with acute respiratory failure who underwent an OLB was more severely ill, had a shorter duration of symptoms and half of the patients fulfilled the diagnostic criteria for ARDS. These patients had a higher mortality rate than those admitted after an elective OLB. This finding was expected as a retrospective study showed that the 28-day mortality in kidney transplant patients with ARDS can reach up to 52.1% (56). These findings suggest that, for patients with acute respiratory failure, invasive diagnostic techniques should be conducted early in the clinical course before the patient’s condition progresses to a point where such procedures can no longer be performed. The OLB is justified to enhance the chance for effective therapy and to minimize side effects (6). Although we did not assess the impact of an OLB on mortality, survival improved in ARDS patients when an OLB led to definitive diagnoses and changes in the treatment (57). It is important to note that patients with specific diagnoses had lower mortality rates in our study, although there was no statistical significance.
Our study has some strength. In contrast to other studies that evaluated the role of OLBs in a heterogeneous group of immunosuppressed patients, we included a consecutive larger sample of kidney transplant patients. We studied two different groups of patients, which included patients who electively underwent an OLB and those in the ICU with acute respiratory failure. However, there are also several limitations. First, our study is a single center and retrospective study. Second, the preliminary investigation of pulmonary disease and the indication for an OLB was not standardized. We do not know the reasons why some patients were submitted to OLB before been submitted to bronchoscopy. In settings with widespread use of molecular diagnostics tools along with a systematic use of flexible bronchoscopy, the type of patient who still has a non-diagnostic workup and proceeds to OLB might be quite different, with different diagnostic yield and procedural risk. Thus, the generalizability of our results may be limited. Third, we did not have a control group of patients in whom empirical treatment was continued without an OLB or a control group of patients submitted only to flexible bronchoscopy. Thus, our paper was not able to address a common clinical conundrum, namely: should kidney transplant patient with diffuse lung disease go to open lung biopsy (OLB)? Fourth, a minimally invasive form of thoracic surgery (percutaneous transthoracic lung biopsy or video assisted thoracoscopic surgery) was not evaluated in our study.
Conclusions
In conclusion, the aspect most worth highlighting in our study is the risk associated with OLB in kidney transplant patients with diffuse pulmonary infiltrates, who did not have definitive diagnoses, and who failed to respond to empiric treatment. The complications related to the procedure were common and were independently associated with intra-hospital mortality. This is particularly relevant in settings where flexible bronchoscopy is not performed systematically prior to OLB. As expected, OLB has high diagnostic yield in this population.
Acknowledgements
We would like to thank the ICU physicians for their assistance during data collection.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethics committee (Comitê de Ética em Pesquisa – Universidade Federal de São Paulo) (No. 125.965) and waived the need for informed consent.
The diagnostic yield and complications of open lung biopsies in kidney transplant patients with pulmonary disease—routine diagnostic tests used to investigate infectious lung diseases
The indication for OLB is not written in our center’s protocol. However, invasive diagnostic methods are usually indicated for all patients with deteriorating clinical or radiologic conditions despite previous investigation and empirical wide-spectrum antibiotics*. Preoperative workup includes the following exams:
- Blood culture;
- Sputum (or suctioned secretions) culture;
- Sputum (or suctioned secretions) samples examined for tuberculosis, fungi, and helminths;
- Legionella urinary antigen test;
- Immunofluorescence for respiratory viruses;
- PCR for Influenza A and B;
- PCR and antigenemia for CMV;
- Serum cryptococcal antigen;
- Serological tests to detect fungal infections.
Fiber-optic bronchoscopy with BAL and transbronchial biopsy is the first invasive diagnostic method option. Open lung biopsy is reserved for cases in which the attending physician judge that flexible bronchoscopy would have a low diagnostic yield or bronchoscopy is not indicated because of the localization of the lesions, or because of the presence of conditions that could increase the risk of complications (high levels of positive end-expiratory pressure, risk of bleeding, and pulmonary hypertension).
*, empirical antibiotic therapy is based on clinical and radiological presentation, epidemiological factors, and previous prophylactic treatments.
References
- Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American Journal of Transplantation 2011;11:2093-109. [Crossref]
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004;351:2715-29. [Crossref]
- Soulillou JP, Giral M. Controlling the incidence of infection and malignancy by modifying immunosuppression. Transplantation 2001;72:S89-93.
- Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med 2005;352:1371-3. [Crossref]
- Karam S, Wali RK. Current State of Immunosuppression: Past, Present, and Future. Crit Rev Eukaryot Gene Expr 2015;25:113-34. [Crossref]
- Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am 2010;24:273-83. [Crossref]
- Canet E, Osman D, Lambert J, et al. Acute respiratory failure in kidney transplant recipients: a multicenter study. Crit Care 2011;15:R91. [Crossref]
- Djamali A, Samaniego M, Muth B, et al. Medical care of kidney transplant recipients after the first posttransplant year. Clin J Am Soc Nephrol 2006;1:623-40. [Crossref]
- Pencheva V, Petrova D, Genov D, et al. Pulmonary Complications as a Cause of Death after Renal Transplantation. Open Journal of Internal Medicine 2014;4:41-6. [Crossref]
- de Carvalho MA, Freitas FG, Silva HT Junior, et al. Mortality predictors in renal transplant recipients with severe sepsis and septic shock. PLoS One 2014;9:e111610. [Crossref]
- Bige N, Zafrani L, Lambert J, et al. Severe infections requiring intensive care unit admission in kidney transplant recipients: impact on graft outcome. Transpl Infect Dis 2014;16:588-96. [Crossref]
- Bafi AT, Tomotani DY, de Freitas FG. Sepsis in Solid-Organ Transplant Patients. Shock 2017;47:12-6. [Crossref]
- Candan S, Pirat A, Varol G, et al. Respiratory problems in renal transplant recipients admitted to intensive care during long-term follow-up. Transplant Proc 2006;38:1354-6. [Crossref]
- Kirby S, Satoskar A, Brodsky S, et al. Histological spectrum of pulmonary manifestations in kidney transplant recipients on sirolimus inclusive immunosuppressive regimens. Diagn Pathol 2012;7:25. [Crossref]
- Pham PT, Pham PC, Danovitch GM, et al. Sirolimus-associated pulmonary toxicity. Transplantation 2004;77:1215-20. [Crossref]
- Defranchi S, Bertolotti AM, Vigliano CA, et al. Open lung biopsy for diffuse disease in patients with and without previously transplanted solid organs. The Annals of Thoracic Surgery 2010;90:965-71; discussion 71-2. [Crossref]
- Stefanutti D, Morais L, Fournet JC, et al. Value of open lung biopsy in immunocompromised children. J Pediatr 2000;137:165-71. [Crossref]
- Fishbein MC. Diagnosis: to biopsy or not to biopsy: assessing the role of surgical lung biopsy in the diagnosis of idiopathic pulmonary fibrosis. Chest 2005;128:520S-525S. [Crossref]
- Thomas JH, Farek PE, Hermreck AS, et al. Diagnostic value of open lung biopsy in immunocompromised patients. Am J Surg 1987;154:692-5. [Crossref]
- Cockerill FR 3rd, Wilson WR, Carpenter HA, et al. Open lung biopsy in immunocompromised patients. Arch Intern Med 1985;145:1398-404. [Crossref]
- Raghu G, Brown KK. Interstitial lung disease: clinical evaluation and keys to an accurate diagnosis. Clin Chest Med 2004;25:409-19. v. [Crossref]
- Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175-9. [Crossref]
- Park JH, Kim DK, Kim DS, et al. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardiothorac Surg 2007;31:1115-9. [Crossref]
- Goldstein G, Keller N, Bilik R, et al. Do immunocompromised children benefit from having surgical lung biopsy performed? Acta Haematol 2015;133:205-9. [Crossref]
- Medina-Pestana JO. More than 1,000 kidney transplants in a single year by the "Hospital do Rim" Group in Sao Paulo - Brazil. Clin Transpl 2010.107-26.
- Ryu JH, Olson EJ, Midthun DE, et al. Diagnostic approach to the patient with diffuse lung disease. Mayo Clin Proc 2002;77:1221-7. [Crossref]
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63 Suppl 5:v1-58. [Crossref]
- McKenna RJ Jr, Mountain CF, McMurtrey MJ. Open lung biopsy in immunocompromised patients. Chest 1984;86:671-4. [Crossref]
- Barbas CS, Capelozzi VL, Hoelz C, et al. Impact of open lung biopsy on refractory acute respiratory failure. J Bras Pneumol 2006;32:418-23. [Crossref]
- Baumann HJ, Kluge S, Balke L, et al. Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery 2008;143:426-33. [Crossref]
- Chuang ML, Lin IF, Tsai YH, et al. The utility of open lung biopsy in patients with diffuse pulmonary infiltrates as related to respiratory distress, its impact on decision making by urgent intervention, and the diagnostic accuracy based on the biopsy location. Journal of intensive care medicine 2003;18:21-8. [Crossref]
- Flabouris A, Myburgh J. The utility of open lung biopsy in patients requiring mechanical ventilation. Chest 1999;115:811-7. [Crossref]
- Kao KC, Tsai YH, Wu YK, et al. Open lung biopsy in early-stage acute respiratory distress syndrome. Crit Care 2006;10:R106. [Crossref]
- Patel SR, Karmpaliotis D, Ayas NT, et al. The role of open-lung biopsy in ARDS. Chest 2004;125:197-202. [Crossref]
- Warner DO, Warner MA, Divertie MB. Open lung biopsy in patients with diffuse pulmonary infiltrates and acute respiratory failure. Am Rev Respir Dis 1988;137:90-4. [Crossref]
- Cheson BD, Samlowski WE, Tang TT, et al. Value of open-lung biopsy in 87 immunocompromised patients with pulmonary infiltrates. Cancer 1985;55:453-9. [Crossref]
- Mason WH, Siegel SE, Tucker BL. Diagnostic open lung biopsy for diffuse pulmonary disease in immunocompromised pediatric patients. Am J Pediatr Hematol Oncol 1982;4:355-9. [Crossref]
- Ellis ME, Spence D, Bouchama A, et al. Open lung biopsy provides a higher and more specific diagnostic yield compared to broncho-alveolar lavage in immunocompromised patients. Fungal Study Group. Scand J Infect Dis 1995;27:157-62. [Crossref]
- Charbonney E, Robert J, Pache JC, et al. Impact of bedside open lung biopsies on the management of mechanically ventilated immunocompromised patients with acute respiratory distress syndrome of unknown etiology. J Crit Care 2009;24:122-8. [Crossref]
- Lee CH, Lee JM, Hung CC, et al. The impact of open lung biopsy on diffuse pulmonary infiltrates in patients with AIDS. Am Surg 2009;75:157-62.
- Lettieri CJ, Veerappan GR, Helman DL, et al. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest 2005;127:1600-5. [Crossref]
- Batista MV, Pierrotti LC, Abdala E, et al. Endemic and opportunistic infections in Brazilian solid organ transplant recipients. Trop Med Int Health 2011;16:1134-42. [Crossref]
- Zihlif M, Khanchandani G, Ahmed HP, et al. Surgical lung biopsy in patients with hematological malignancy or hematopoietic stem cell transplantation and unexplained pulmonary infiltrates: improved outcome with specific diagnosis. Am J Hematol 2005;78:94-9. [Crossref]
- Naiditch JA, Barsness KA, Rothstein DH. The utility of surgical lung biopsy in immunocompromised children. J Pediatr 2013;162:133-6.e1. [Crossref]
- Georgiadou SP, Sampsonas FL, Rice D, et al. Open-lung biopsy in patients with undiagnosed lung lesions referred at a tertiary cancer center is safe and reveals noncancerous, noninfectious entities as the most common diagnoses. Eur J Clin Microbiol Infect Dis 2013;32:101-5. [Crossref]
- Cheng GS, Stednick ZJ, Madtes DK, et al. Decline in the Use of Surgical Biopsy for Diagnosis of Pulmonary Disease in Hematopoietic Cell Transplantation Recipients in an Era of Improved Diagnostics and Empirical Therapy. Biol Blood Marrow Transplant 2016;22:2243-2249. [Crossref]
- Harris B, Lowy FD, Stover DE, et al. Diagnostic bronchoscopy in solid-organ and hematopoietic stem cell transplantation. Ann Am Thorac Soc 2013;10:39-49. [Crossref]
- Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712-22. [Crossref]
- Hoyo I, Linares L, Cervera C, et al. Epidemiology of pneumonia in kidney transplantation. Transplant Proc 2010;42:2938-40. [Crossref]
- Kupeli E, Akcay S, Ulubay G, et al. Diagnostic utility of flexible bronchoscopy in recipients of solid organ transplants. Transplant Proc 2011;43:543-6. [Crossref]
- Springmeyer SC, Silvestri RC, Sale GE, et al. The role of transbronchial biopsy for the diagnosis of diffuse pneumonias in immunocompromised marrow transplant recipients. Am Rev Respir Dis 1982;126:763-5.
- Walker WA, Cole FH Jr, Khandekar A, et al. Does open lung biopsy affect treatment in patients with diffuse pulmonary infiltrates? J Thorac Cardiovasc Surg 1989;97:534-40.
- Chaparro C, Maurer JR, Chamberlain DW, et al. Role of open lung biopsy for diagnosis in lung transplant recipients: ten-year experience. Ann Thorac Surg 1995;59:928-32. [Crossref]
- Camargo LF, Gomes PS, Machado PG, et al. Management of Pulmonary Infections in Kidney Transplant Patients. Clinical Pulmonary Medicine 2003;10:308-21. [Crossref]
- Libby LJ, Gelbman BD, Altorki NK, et al. Surgical lung biopsy in adult respiratory distress syndrome: a meta-analysis. Ann Thorac Surg 2014;98:1254-60. [Crossref]
- Shorr AF, Abbott KC, Agadoa LY. Acute respiratory distress syndrome after kidney transplantation: epidemiology, risk factors, and outcomes. Crit Care Med 2003;31:1325-30. [Crossref]
- Papazian L, Doddoli C, Chetaille B, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med 2007;35:755-62. [Crossref]