Thoracoabdominal aortic replacement for Crawford extent II aneurysm after thoracic endovascular aortic repair
Introduction
Thoracic endovascular aortic repair (TEVAR) was first introduced to treat aortic dissection in 1999 (1). It has since achieved wide acceptance for the treatment of type B aortic dissection, because of its lower mortality and morbidity rates compared with conventional open repair. However, although the short- and mid-term results of TEVAR are good, the long-term results remain unsatisfactory as a result of serious complications, such as endoleaks, retrograde type A dissection, residual aneurysm enlargement, and rupture (2-5). Re-intervention is typically endovascular, but patients with certain complications such as retrograde type A dissection and residual aneurysm enlargement are often ultimately converted to open repair (6). Although selected high-volume centers have achieved good results for open thoracoabdominal aortic aneurysm (TAAA) repair (7), the treatment of Crawford extent II aneurysm (dissection-related TAAA) after TEVAR remains challenging because of the need to remove the failed endograft, and the complexity of the aortic reconstruction. In this study, we reviewed our experience with surgical management of Crawford extent II aneurysms after TEVAR using thoracoabdominal aortic replacement (TAAR).
Methods
Patients
Eleven patients (10 male, 1 female) with Crawford extent II aneurysm after TEVAR underwent TAAR at Beijing Aortic Disease Center, Beijing Anzhen Hospital between August 2012 and May 2015. Surgery was performed in five patients under deep hypothermic cardiac arrest, in two under mild hypothermic cardiopulmonary bypass (CPB), and in four under direct aortic cross-clamping at normal temperature. This study was approved by the institutional review board of Capital Medical University (No. 2016011X). The mean age of the patients was 43±6 (range, 32–55) years. The mean interval between TEVAR and TAAR was 29±20 (range, 6–72) months. The largest diameter of the descending aorta was 62±15 (range, 40–90) mm. A history of previous aortic surgery was seen in 5 (Table 1), hypertension in 7, and Marfan syndrome in 2. Aortic dissection was confirmed by contrast-enhanced computed tomography prior to surgery.

Full table
Surgical technique
The basic surgical technique for open repair of extensive TAAA has been described in detail elsewhere (8). If the stent needed to be removed, the procedure was carried out on pump. CPB was established through the left femoral artery and vein. Once the nasopharyngeal temperature was cooled to 22–25 °C and the heart was arrested, the descending aorta beyond the stent was cross-clamped, the proximal part of the descending aorta was incised, and the previously inserted stent was removed. A four branch graft with two 10-mm SideArms and two 8-mm SideArms was used in the operation. The proximal suture edge (usually at the level of the left subclavian artery orifice) was trimmed and anastomosed to the main side of the graft. A 10-mm sidearm was then used as arterial line to resume CPB, and rewarming was initiated. The intercostal arteries were identified, and one 8 mm sidearm was used to restore blood flow to the intercostal artery in a “patch-to-patch” fashion. The left renal artery was anastomosed to the other 8 mm sidearm in an “end-to-end” fashion. The celiac artery, superior mesenteric artery, and right renal artery were then trimmed as an island patch and attached to the other side of the main graft. One 10-mm sidearm was attached to left iliac artery in an “end-to-side” fashion. Thereafter, the arterial inflow to the 10-mm sidearm was discontinued, and the sidearm was attached to the right iliac artery in an “end-to-side” fashion. CPB was then discontinued. If the stented portion of the descending aorta could be cross-clamped, cardiac arrest was not deemed necessary, and the procedure was carried out off-pump. In such cases, we initially attached a 10-mm sidearm to the respective iliac artery, and the descending aorta was then cross-clamped beyond the distal end of the stent and to include the portion with the stent. The aorta between the cross-clamps was opened, the distal end of the stent was trimmed, a felt strip was positioned inside the stent, and the aortic wall, stent, and felt were sewn together to the main side of the graft. The cross-clamped stented portion was removed, and all the organs except the incised portion were perfused. The following procedures could then be carried out in a segmental cross-clamp fashion (9). Data on the surgical procedures are listed in Table 2.
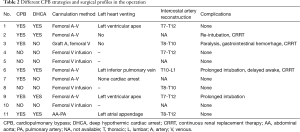
Full table
Follow-up
Clinical data were obtained by personal and telephone interviews with patients, family members, and primary care physicians. Complications such as neurologic, renal, and respiratory morbidities were recorded. Contrast-enhanced computed tomography was performed before discharge, at 3 and 6 months postoperatively, and annually thereafter.
Results
Surgical data
The mean operation time was 627±86 (range, 420–735) min. Seven patients underwent CPB during the operation, including five who received deep hypothermic circulatory arrest. The remaining four patients underwent surgery off-pump at normal temperature. CPB time (seven cases) was 170±89 (range, 32–258) min, deep hypothermic circulatory arrest time (five cases) was 34±23 (range, 19–74) min. The estimated blood loss during surgery was 3,600±2,110 (range, 800–9,000) mL, and the average red blood cell infusion during surgery was 9.5±6.3 (range, 0–20) U. The mean duration of ventilation was 28±38 (range, 10–133) h, and the mean length of intensive care unit stay was 102±117 (range, 14–349) h.
Morbidity and mortality
There were no in-hospital deaths. One patient sustained paraplegia after surgery, but recovered partially when discharged, and completely during follow-up. Temporary (<4 days) continuous renal replacement therapy was required in 3 patients for acute renal insufficiency. Re-intubation was needed in 1 patient due to respiratory failure, and two patients had prolonged intubation with transient cerebral ischemia. One patient experienced gastrointestinal hemorrhage but recovered uneventfully.
All patients were followed-up or 21.6±10.3 months (range 10–42). There was one death during follow-up, but no cases of late spinal cord injury (SCI) or visceral organ ischemia occurred. All but one of the patients resumed normal activities and received antihypertensive therapy after hospital discharge.
Discussion
The use of TEVAR to treat aortic dissection was first reported in 1999 (1). Although its favorable short- and mid-term results has gained wider acceptance for TEVAR in treating type B aortic dissection, its long-term results remain unsatisfactory because of serious complications (10), such as endoleaks, retrograde type A dissection, and residual aneurysm enlargement. Open surgical repair is required because of the failure or unsuitability of endografting in patients with extensive dilatation of the thoracoabdominal aorta. The reasons for failed TEVAR in these patients include a short proximal and/or distal landing zone, steep arch angulation, aneurysm formation distal to the stent-graft, and connective tissue disease. Managing Crawford extent II aneurysms after stent-graft failure is extremely challenging because of the need to remove the failed endograft, the technical complexity of the aortic reconstruction, and the extensive surgical trauma.
Several factors may make the procedure even more difficult in these patients. First, the visceral arterial part of the descending aorta is usually expanded, and these visceral arteries are therefore compressed and adhering to adjacent tissues, thus making them difficult to dissect. Second, most of the upper thoracic part of the intercostal arteries are occluded because of the previous endograft, rendering reconstruction of enough intercostal arteries impossible. Third, the stent needs to be removed if the stented part of the descending aorta is expanded.
Historically, Crawford extent II aneurysms typically necessitated open surgical repair, which is a time-consuming procedure requiring the reconstruction of numerous visceral arteries. Crawford (11) introduced three types of visceral artery reconstructions in the early 1970s, of which type II (when aorta graft was in position, an elliptical segment of the anterior medial part of graft circumference was removed, leaving an opening large enough for suture around that part of aortic wall containing the origins of the right renal, superior mesenteric, and celiac axis, the left renal arteries was attached to the side of the aortic graft) was considered to be preferable.
The visceral arteries in post-TEVAR patients are usually dissection related, compressed, and show more adhesion than those in patients without prior TEVAR, making their dissection more difficult. In our institution, we used a four-branched Dacron graft and applied a “modified type II” reconstruction of the visceral arteries, in which the right renal artery, superior mesenteric artery, and celiac axis were trimmed as a single patch, and anastomosed to one main side of the graft, and the left renal artery was attached to an 8-mm sidearm in an end-to-end fashion, thus reducing the time required for visceral artery dissection and reconstruction. Although patch aneurysms are not infrequent complications of TAAR, especially in patients with connective tissue disorders such as Marfan syndrome (12,13), no patch aneurysm was confirmed during follow-up in the current study.
The management of proximal anastomosis during this procedure is technically difficult. The proximal end of the stent applied in TEVAR is usually located at the level of the left subclavian artery (LSCA) orifice, sometimes to the level of the left common carotid artery (LCCA) orifice. If the stent was removed, the proximal anastomosis level would be located as proximal as the LSCA orifice, and the LSCA and aortic arch between the LSCA and LCCA need to be cross-clamped separately. However, this may not be possible, should deep hypothermic circulatory arrest be necessary. In this setting, the aortic wall may also be abnormal, predisposing the anastomosis to a high risk of bleeding. If the stent was left in situ, the stent, aortic wall, and a felt strip were all sewn to the main graft. However, because the two sides are not matched in terms of size and thickness, anastomotic bleeding is also common, and further reinforcement of the anastomosis may be required. Fortunately, however, no anastomotic bleeding occurred in our cohort.
SCI is a catastropic complication following TAAR, and despite improved perfusion strategies such as cerebrospinal fluid drainage and active cooling, the risk of paraplegia is still not negligible (14). Patients who have undergone prior TEVAR are theoretically at increased risk, because the previous TEVAR may have covered most of the intercostal arteries (15), making them unsuitable for reconstruction. Intercostal artery reconstruction is known to be important for preventing SCI, and failure to reconstruct them may thus predispose patients to SCI, potentially resulting in paraplegia.
In our study, seven patients underwent intercostal artery reconstruction, while four did not because there were no target intercostal arteries. Postoperatively, one patient developed paralysis, and he had recovered partially at discharge, and recovered completely during follow-up. None of the four patients without intercostal artery reconstruction during TAAR suffered from paraplegia. Several factors might contribute to this favorable outcome. First, the spinal cord blood supply depends on the internal iliac artery, intercostal arteries, and anterior spinal cord artery, which originate from the subclavian artery. In our patients, the LSCA and internal iliac arteries were intact, and these blood supplies were not damaged. Second, the interval between TEVAR and TAAR is long enough for the collateral arteries to regenerate. Third, the strategy we applied minimized the duration of spinal cord ischemia.
Acute renal insufficiency is another common complication of TAAR, and although some patients may recover completely, others may not (16). In our study, three patients experienced acute renal insufficiency and required temporary continuous renal replacement therapy, which was discontinued in all cases after 4 days.
Previous studies demonstrated long-term outcomes following TAAR for chronic type B dissection, including operative death in 22.6%, with an in-hospital mortality of 16.3% in elective patients and 36.8% in non-elective patients. Bashir reported that the incidence was 3.2% for permanent paraplegia or paraparesis, 11.3% for stroke, and 25.8% for renal insufficiency requiring dialysis (17). In other reports, the incidence ranged from 3.4–8.9% for 30-day mortality, 3.4–12.3% for in-hospital mortality, 1.5–5.8% for paraplegia, 3.7–6.3% for stroke, and 1.7–14.3% for renal failure (18,19).
Despite the high morbidity and mortality associated with surgical management of Crawford type II aneurysms, and the difficulties in managing Crawford type II aneurysms after TEVAR, the results in the present study are encouraging, with no in-hospital deaths and only one death during follow-up, and no case of late SCI or visceral ischemia. All but one of the patients resumed normal activities after hospital discharge.
Re-TEVAR, fenestrated and branched (Fe/Br) endovascular aortic repair, and hybrid operations are alternatives to open surgical repair of thoracoabdominal aortic diseases. Fe/Br endovascular aortic repair is an established treatment for non-dissected TAAA (20), but the potential for true lumen stent graft compression, inadequate end-organ blood flow to visceral vessels supplied by the false lumen, and more challenging technical issues with implantation represent the major in patients with dissection repair (21). Hybrid operations that combine both open (typically visceral/renal de-branching) and endovascular techniques were first reported in 1999 (22). Although they theoretically have the potential to decrease overall morbidity by avoiding thoracotomy, potential paralysis of the left hemi-diaphragm, and cross-clamping of the aorta, they were associated with a mortality of up to 20% in literature (23).
Limitation
The present study had some limitations. First, it was a retrospective study; second, Fe/Br is not applied in our institution, and there was therefore a lack of comparable data for open surgical repair and Fe/Br; third, the sample size was small, with only 11 cases included in the current study.
Conclusions
The results of the present study suggest that TAAR is a feasible option with encouraging results in patients with Crawford extent II aneurysms after TEVAR.
Acknowledgements
Funding: This study was supported by grants from the special research fund for public welfare industry of Health from National Health and Family planning Commission of China (No. 201402009) and The National Key Technology R&D Program (No. 2015BAI12B03).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Capital Medical University (No. 2016011X).
References
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546-52. [Crossref]
- Geisbüsch P, Hoffmann S, Kotelis D, et al. Reinterventions during midterm follow-up after endovascular treatment of thoracic aortic disease. J Vasc Surg 2011;53:1528-33. [Crossref]
- Li B, Pan XD, Ma WG, et al. Stented elephant trunk technique for retrograde type A aortic dissection after endovascular stent graft repair. Ann Thorac Surg 2014;97:596-602. [Crossref]
- Canaud L, Alric P, Gandet T, et al. Open surgical secondary procedures after thoracic endovascular aortic repair. Eur J Vasc Endovasc Surg 2013;46:667-74. [Crossref]
- Dumfarth J, Michel M, Schmidli J, et al. Mechanisms of failure and outcome of secondary surgical interventions after thoracic endovascular aortic repair (TEVAR). Ann Thorac Surg 2011;91:1141-6. [Crossref]
- Patterson B, Holt P, Nienaber C, et al. Aortic pathology determines midterm outcome after endovascular repair of the thoracic aorta: report from the Medtronic Thoracic Endovascular Registry (MOTHER) database. Circulation 2013;127:24-32. [Crossref]
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4; discussion S890-2.
- Cheng L, Huang F, Chang Q, et al. Repair of extensive thoracoabdominal aortic aneurysm with a tetrafurcate graft: midterm results of 63 cases. Heart Surg Forum 2010;13:E1-6. [Crossref]
- Frank SM, Parker SD, Rock P, et al. Moderate hypothermia, with partial bypass and segmental sequential repair for thoracoabdominal aortic aneurysm. J Vasc Surg 1994;19:687-97. [Crossref]
- Hanna JM, Andersen ND, Ganapathi AM, et al. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg 2014;59:96-106. [Crossref]
- Crawford ES. Thoraco-abdominal and abdominal aortic aneurysms involving renal, superior mesenteric, celiac arteries. Ann Surg 1974;179:763-72. [Crossref]
- Tshomba Y, Melissano G, Civilini E, et al. Fate of the visceral aortic patch after thoracoabdominal aortic repair. Eur J Vasc Endovasc Surg 2005;29:383-9. [Crossref]
- Kulik A, Allen BT, Kouchoukos NT. Incidence and management of intercostal patch aneurysms after repair of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2009;138:352-8. [Crossref]
- Tian DH, De Silva RP, Wang T, et al. Open surgical repair for chronic type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014;3:340-50.
- Maeda T, Yoshitani K, Sato S, et al. Spinal cord ischemia after endovascular aortic repair versus open surgical repair for descending thoracic and thoracoabdominal aortic aneurism. J Anesth 2012;26:805-11. [Crossref]
- Johns N, Jamieson RW, Ceresa C, et al. Contemporary outcomes of open repair of thoracoabdominal aortic aneurysm in young patients. J Cardiothorac Surg 2014;9:195. [Crossref]
- Bashir M, Shaw M, Fok M, et al. Long-term outcomes in thoracoabdominal aortic aneurysm repair for chronic type B dissection. Ann Cardiothorac Surg 2014;3:385-92.
- LeMaire SA, Price MD, Green SY, et al. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg 2012;1:286-92.
- Di Luozzo G, Geisbüsch S, Lin HM, et al. Open repair of descending and thoracoabdominal aortic aneurysms and dissections in patients aged younger than 60 years: superior to endovascular repair? Ann Thorac Surg 2013;95:12-9; discussion 19. [Crossref]
- Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg 2010;140:S171-8. [Crossref]
- Kasirajan K, Milner R, Chaikof EL. Late complications of thoracic endografts. J Vasc Surg 2006;43 Suppl A:94A-99A.
- Quiñones-Baldrich WJ, Panetta TF, Vescera CL, et al. Repair of type IV thoracoabdominal aneurysm with a combined endovascular and surgical approach. J Vasc Surg 1999;30:555-60. [Crossref]
- Resch TA, Greenberg RK, Lyden SP, et al. Combined staged procedures for the treatment of thoracoabdominal aneurysms. J Endovasc Ther 2006;13:481-9. [Crossref]


