Minimally invasive surgery for giant esophageal leiomyoma: a case report & review of the literatures
Introduction
Leiomyoma is the most common benign esophageal neoplasm. The size of the lesion may not change for many years. However, some esophageal leiomyomas become giant (diameter of tumor larger than 10 cm) gradually and show symptoms caused by compression of tumor, obstruction of esophagus or dysfunction of cardia (1,2).
Surgical treatment has traditionally been the therapy of choice for esophageal leiomyoma, including thoracotomy, thoracoscopic approach, endoscopic approach and esophageal resection (3). Despite the rapid development of minimally invasive surgery, the indication for thoracoscopic approach is still controversial. Giant tumors are found difficult for surgeons to develop the plane between the tumor and underlying submucosa and small tumors are not good candidates for thoracoscopic enucleation as well, because tumors of small size are difficult to be localized under thoracoscopy (4).
We report a case of giant esophageal leiomyoma which was successfully enucleated by using VATS and review published cases of giant leiomyomas in the past ten years.
Case presentation
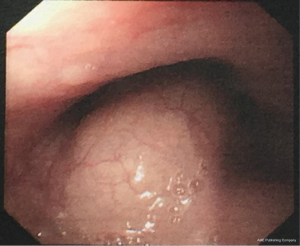
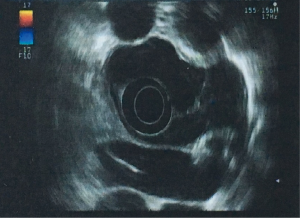
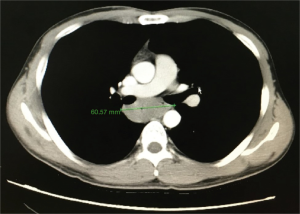
A 29-year-old man was admitted to the clinic for the complaints of 2-month history of progressive dysphagia and discomfort to solids. Upper gastrointestinal endoscopy revealed a mucosal protuberant lesion at 25–30 cm from the incisor teeth and a normal, smooth esophageal mucosa (Figure 1). Endoscopic ultrasonography (EUS) revealed the well-circumscribed, hypoechoic mass originated from the muscularis propria layer (Figure 2). Computed tomography (CT) of the thorax showed a well-circumscribed soft tissue opacity with homogeneous density located in the middle and lower thirds of the thoracic esophagus (Figure 3).
Definitive diagnosis can be made only by histologic examination, but no endoscopic biopsy was performed in the current case. The symptomatic patient underwent enucleation of the tumor by using VATS.
The patient was intubated with a double-lumen endotracheal tube to allow single lung ventilation. He was placed in left lateral decubitus position. One 1.0 cm incision was made in the seventh intercostal space along the right middle axillary line, and thoracoscopy was introduced through the incision. For the operation, one 0.5-cm incision was made in the fourth intercostal space along the right anterior axillary line, and another 0.5-cm incision was made in the ninth intercostal space along the right posterior axillary line. Exploration revealed an irregular tumor located below the carina level. The muscularis of the esophagus was cut to expose the capsule of the tumor and a well-defined solid tumor with an intact capsule was revealed (Figure 4). The plane between the submucosa and the tumor was developed by blunt dissection. The esophageal mucosal layer was carefully preserved during the operation and an air-water test was performed to check the integrity of the mucosa. The esophageal muscular layer and the mediastinal pleura were sutured by using poly filament absorbable 3/0 suture material. The incision at the 0.5-cm trocar site was extended to 2 cm and the tumor was removed in a specimen bag.
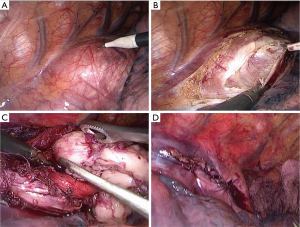
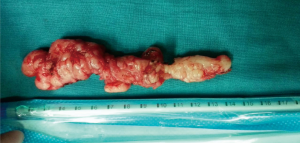
The tumor was approximately 15 cm × 2.5 cm × 1.5 cm in size, with a lobulated ellipsoidal shape (Figure 5). Following control of hemostasis, a latex drainage tubing was inserted to the esophageal bed in the ninth intercostal space along the posterior axillary line and a chest tube was inserted in the seventh intercostal space along the anterior axillary line. Estimated intraoperative blood loss was 20 mL.
The tumor was enucleated without mucosal damage The patient started fluid diet on the 1st postoperative day, and then semifluid diet on the 2nd postoperative day. The patient resumed intake of normal diet on the 5th postoperative day. We performed a radiological control of the upper digestive tract with contrast medium on the 5th postoperative day to examine the recovery of gastrointestinal function after surgery and it revealed satisfactory digestive system recovery. The chest tubes were removed on the 4th postoperative day. Although no postoperative complications such as esophageal leakage and wound infection occurred, the patient had a low fever after the surgery and lasted for several days. For this reason, we prolonged the patient’s hospital stay until his temperature returned to normal level and the patient was discharged on the 8th postoperative day. The VATS enucleation achieved remission of his symptoms.
Discussion
The present case report describes a patient with a giant, symptomatic leiomyoma of the esophagus which was enucleated using VATS. The tumor was approximately 15 cm × 2.5 cm × 1.5 cm in size and it was enucleated without mucosal damage. The patient resumed oral intake on the 1st postoperative day, with following solid meal and the patient was discharged on the 8th postoperative day in the absence of any postoperative complications.
Esophageal leiomyomas greater than 10 cm in diameter are generally designated as giant leiomyomas (4,5). However, the definition of “giant leiomyoma” seems vague in clinical practice. The size varies from 5.2 to 22.5 cm according to the previous publications (5-17) (Table 1). Giant esophageal leiomyoma is traditionally treated by open thoracotomy (10). Minimally invasive surgery is considered not quite appropriate for giant tumor because of the difficulty in developing the plane between the tumor and underlying submucosa. Recently, some centers implemented minimally invasive surgeries for the treatment of giant esophageal leiomyoma, including resection or enucleation of esophageal leiomyoma by thoracoscopy, laparoscopy or Da Vinci robot-assisted thoracoscopy (6,8,10-13,15-17). In spite of the rapid development of minimally invasive surgery, the indication for thoracoscopic approach remains controversial, which depends on the size and location of the tumor and experience of the surgeon. According to the previous publications (Table 1), open thoracotomy approach was applied in esophageal leiomyoma whose size vary from 8 to 17 cm (5,7,9,14), and minimally invasive surgery was applied in esophageal leiomyoma whose size vary from 9 to 22.5 cm (6,8,10,11,13,17). Therefore, the giant size of tumor isn’t an absolute restriction for minimally invasive approach any longer, and the experience and technique of the surgeon plays a more important role in determining surgery approach.
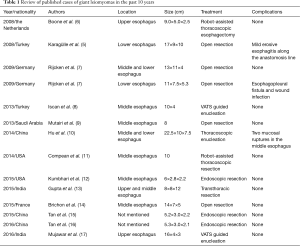
Full table
The well-circumscribed growth feature of esophageal leiomyomas facilitates minimally invasive approach to be performed under thoracoscopy. Compared to open resection, minimally invasive surgery has the advantages in precision, flexibility and control, which facilitates the surgeon better conditions to preserve esophageal mucosa. Meanwhile, minimally invasive surgery is supposed to contribute to minimize postoperative complications, length of hospital stay and pain, and bring better cosmetic results.
Generally, complication and postoperative recovery condition need to be taken into consideration when judging the effect of surgical treatment. According to the previous publications, mild erosive esophagitis and esophago pleural fistula arising from the intrathoracic esophagogastrostomy occurred in patients underwent open resection and mucosal ruptures occurred in patients underwent minimally invasive surgery (5,7,10). Because of the absence of operation time, we can’t prove whether minimally invasive approach consumes less time than open resection. In addition, postoperative conditions are unavailable in some cases, so we can’t get direct evidence whether minimally invasive approach is better for patients to recover after surgery. Nevertheless, we have a general impression from the previous publications that patients underwent minimally invasive surgery resume intake of a normal diet and discharge from hospital at an earlier time than those who underwent open resection, which may reflect that minimally invasive approach benefits patients in promoting postoperative recovery (5,6,8,10,11,15-17). In our case, the patient was offered VATS enucleating in the absence of any postoperative complications and resumed oral intake on the 1st postoperative day, which reflects better postoperative condition compared to previous publications.
Laceration of the esophageal mucosa is the most common complication in the process of enucleation (18). And the key in esophageal leiomyoma surgery is to preserve the esophageal mucosa. Since preoperative endoscopic biopsy may result in the development of fibrosis between the tumor and the submucosa layer, which significantly increases the risk of mucosal perforation during tumor enucleation, it is not recommended to patients with esophageal leiomyoma (19,20).
A report by Ozdil et al. described treatment of giant leiomyoma with endoscopic percutaneous injection of ethanol (21). This method is reported to be a safe alternative to surgery for the treatment of large gastric leiomyomas which appears to be more applicable and less invasive. However, most of the esophageal leiomyomas treated in this manner are smaller than 10 cm and the effect of ethanol injection therapy is not comparable to minimally invasive approach due to the limitation of patient numbers.
Likewise, this report has the limitation of patient numbers. More cases need to be collected for further statistical analysis to prove the benefits of minimally invasive surgery in the treatment of giant esophageal leiomyoma.
Conclusions
In conclusion, minimally invasive surgery is a safe and effective approach in the surgical treatment for giant esophageal leiomyomas. And its further efficacy remains to be proved by further clinical evidence with larger samples.
Acknowledgements
I would like to extend my deepest gratitude to my mentor, Dr. Lijie Tan, a respectable, responsible and visionary scholar, who has provided me with valuable guidance in every stage of the writing of this thesis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Seremetis MG, Lyons WS, deGuzman VC, et al. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer 1976;38:2166-77. [Crossref]
- Cheng BC, Chang S, Mao ZF, et al. Surgical treatment of giant esophageal leiomyoma. World J Gastroenterol 2005;11:4258-60. [Crossref]
- Lee LS, Singhal S, Brinster CJ, et al. Current management of esophageal leiomyoma. J Am Coll Surg 2004;198:136-46. [Crossref]
- Jiang G, Zhao H, Yang F, et al. Thoracoscopic enucleation of esophageal leiomyoma: a retrospective study on 40 cases. Dis Esophagus 2009;22:279-83. [Crossref]
- Karagülle E, Akkaya D, Türk E, et al. Giant leiomyoma of the esophagus: a case report and review of the literature. Turk J Gastroenterol 2008;19:180-3.
- Boone J, Draaisma WA, Schipper ME, et al. Robot-assisted thoracoscopic esophagectomy for a giant upper esophageal leiomyoma. Dis Esophagus 2008;21:90-3.
- Rijcken E, Kersting CM, Senninger N, et al. Esophageal resection for giant leiomyoma: report of two cases and a review of the literature. Langenbecks Arch Surg 2009;394:623-9. [Crossref]
- Iscan Y, Tunca F, Senyurek YG, et al. Thoracoscopic enucleation of a giant leiomyoma of the esophagus. Surg Laparosc Endosc Percutan Tech 2013;23:e32-4. [Crossref]
- Mutairi H, Al-Akkad M, Afzal M, et al. Giant leiomyoma of the oesophagus with eosinophilic infiltration. BMJ Case Rep 2013;2013. pii: bcr2013201343.
- Hu X, Lee H. Complete thoracoscopic enucleation of giant leiomyoma of the esophagus: a case report and review of the literature. J Cardiothorac Surg 2014;9:34. [Crossref]
- Compean SD, Gaur P, Kim MP. Robot assisted thoracoscopic resection of giant esophageal leiomyoma. Int J Surg Case Rep 2014;5:1132-4. [Crossref]
- Kumbhari V, Saxena P, Azola A, et al. Submucosal tunneling endoscopic resection of a giant esophageal leiomyoma. Gastrointest Endosc 2015;81:219-20. [Crossref]
- Gupta V, Sinha SK, Vaiphei K, et al. Esophageal resection for giant leiomyoma. J Cancer Res Ther 2015;11:651. [Crossref]
- Brichon PY, Laverriere MH, Guigard S, et al. A Giant Purely Polypoid Esophageal Leiomyoma. Ann Thorac Surg 2015;100:301-2. [Crossref]
- Tan Y, Liu D. En bloc submucosal tunneling endoscopic resection for a giant esophageal leiomyoma. Gastrointest Endosc 2015;82:399. [Crossref]
- Tan Y, Zhu H, Lv L, et al. Enlarging an accidental mucosotomy to facilitate tumor extraction during submucosal tunneling endoscopic resection for a giant esophageal leiomyoma. Gastrointest Endosc 2016;83:248-9. [Crossref]
- Mujawar P, Pawar T, Chavan RN. Video Assisted Thoracoscopic Surgical Enucleation of a Giant Esophageal Leiomyoma Presenting with Persistent Cough. Case Rep Surg 2016;2016:7453259.
- Saleh WN, Bamosa A, Al-Mutairi H, et al. Thoracoscopic enucleation of esophageal leiomyoma in patient with MEN I syndrome. Ann Thorac Med 2010;5:47-9. [Crossref]
- Herrmann JL, Crisostomo PR, Meldrum DR. Current Therapy in Thoracic and Cardiovascular Surgery. J Surg Res 2009;155:357-8. [Crossref]
- Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc 2003;57:68-72. [Crossref]
- Ozdil B, Akkiz H, Kece C, et al. Endoscopic alcohol injection therapy of giant gastric leiomyomas: an alternative method to surgery. Can J Gastroenterol 2010;24:533-5. [Crossref]