|
Original Article
Comparative outcomes of squamous and non-squamous non-small cell lung cancer (NSCLC) patients in phase II studies of ASA404 (DMXAA) – retrospective analysis of pooled data
Mark J McKeage1, Michael B Jameson2, AS1404-201 Study Group Investigators
1The University of Auckland, Auckland 1023, New Zealand; 2Oncology Department, Waikato Hospital, Hamilton 3240, New Zealand
Corresponding author: Mark J. McKeage, MD. Department of Pharmacology and Clinical Pharmacology, The University of Auckland, 85 Park Road, Private Bag 92019, Grafton, Auckland 1023, New Zealand. Phone: +64-9-3737-599; Fax: +64-9-3737-556. E-mail: m.mckeage@auckland.ac.nz
|
|
Abstract
Background: ASA404 (5,6-dimethylxanthenone-4-acetic acid) is a small-molecule, flavonoid tumor-vascular disrupting agent. Pooled data from phase II studies were analyzed retrospectively to compare safety and efficacy between squamous and non-squamous non-small cell lung cancer (NSCLC) patients.
Methods: Data from previously untreated patients with stage IIIb/IV NSCLC who were randomized to receive up to six cycles of carboplatin (C; AUC 6 mg/ml•min) and paclitaxel (P; 175 mg/m2) alone or with ASA404 (1200 mg/m2), or enrolled in an extension study to receive CP and ASA404 (1800 mg/m2), were analyzed. Differences between subgroups were calculated using Fisher’s exact test.
Results: Of the 108 enrolled patients, safety data from the 104 patients included in the safety population were pooled to compare results between histological subgroups (squamous vs non-squamous) and treatment (CP alone vs CP + ASA404). Addition of ASA404 to the standard chemotherapy regimen did not appear to substantially increase toxicity, and there were no serious adverse events associated with bleeding, pulmonary hemorrhage, or hemoptysis. Activity with CP + ASA404 appeared improved over CP alone, with median survival 10.2 vs 5.5 months in squamous, and 14.9 vs 11.0 months in non-squamous populations, respectively.
Conclusion: This analysis is limited by its retrospective nature, and by the small size of the overall group, treatment and disease subgroups. However, as ASA404 appears to have a similar safety and activity profile in patients with squamous and non-squamous NSCLC, the findings support inclusion of both groups of patients in ongoing definitive phase III trials of ASA404 (NCT00832494).
Key words
ASA404; non-small cell lung carcinoma; clinical trial; phase II; safety
J Thorac Dis 2010; 2: 199-204. DOI: 10.3978/j.issn.2072-1439.2010.02.04.1
|
|
Introduction
Lung cancer is the leading cause of cancer death in the United
States ( 1) and worldwide ( 2). Non-small cell lung cancer
(NSCLC) accounts for about 85% of all lung cancers ( 2), and
can be subclassified as squamous (~30%) or non-squamous
(~70%; includes adenocarcinoma and large cell histologies)
histological types ( 3). Squamous NSCLC is a particularly aggressive form of lung cancer, for which there is a lack of effective and well-tolerated
treatments available. New cytotoxic agents and targeted
therapies have been evaluated, but many show little promise for
first-line therapy of squamous NSCLC. For example, overall
survival with the pemetrexed/cisplatin combination was inferior
to gemcitabine/cisplatin in patients with squamous NSCLC
histology, which was in contrast to the results seen in patients
with some non-squamous forms of the disease ( 4). Furthermore,
certain anti-angiogenic agents, such as bevacizumab, sorafenib
and motesanib, have been associated with safety concerns in
patients with squamous NSCLC, limiting their use to patients
with non-squamous histology only ( 5-7). ASA404 (vadimezan; DMXAA) is a novel, small molecule
flavonoid tumor-vascular disrupting agent (Tumor-VDA) which
targets the existing tumor vasculature, selectively inhibiting
tumor blood flow and causing extensive necrosis of the tumor
core ( 8). A phase II, multicentre, open-label study ( 9), and
single-arm extension study ( 10) evaluated carboplatin and
paclitaxel (CP) in combination with ASA404 (at doses of 1200 mg/m 2 and 1800 mg/m 2) as a first-line treatment for advanced
NSCLC. Patients with both squamous and non-squamous
NSCLC were enrolled. Addition of ASA404 to the standard
chemotherapy regimen did not appear to substantially increase
toxicity. Furthermore, in these two small phase II studies,
ASA404 was associated with improved response rate, median
time to progression (TTP) and median survival compared with
the chemotherapy regimen alone. The current retrospective analysis explores the safety
and activity of ASA404 in combination with standard CP
chemotherapy in patients with squamous and non-squamous
advanced NSCLC using pooled results from phase II evaluations
of ASA404 (1200 and 1800 mg/m 2) ( 9, 10). Although limited by
the small sample size, the objective of this study was to provide
a preliminary indication of the safety and efficacy of ASA404 in
patients with squamous or non-squamous advanced NSCLC to
inform the study design of phase III clinical trials.
|
|
Methods
Detailed methods for the randomized, phase II, multicenter,
open-label study (CP + ASA404 1200 mg/m 2 vs CP alone)
and extension study (CP + ASA404 1800 mg/m 2) have been
published previously ( 9, 10). The core eligibility criteria for inclusion in the study were:
age 18 years or older; histologically confirmed, locally advanced
or metastatic NSCLC (stage IIIb/IV, not curable by surgery
or radiotherapy); one or more unidimensionally measurable
lesions according to the Response Evaluation Criteria in Solid
Tumors (RECIST); and no previous chemotherapy ( 11). Other
requirements included a Karnofsky performance status ≥70%;
a life expectancy of ≥3 months; and adequate hematologic,
renal and hepatic function. Exclusion criteria included major
surgery or radiotherapy (unless palliative) within 4 weeks of
enrollment, CNS metastases, small cell or mixed lung cancer,
pregnancy, use of medication known to affect systemic serotonin
levels or QTc interval, and QTc interval prolongation or cardiac
arrhythmia. There were no restrictions relating specifically
to prior history of hemoptysis, anticoagulant therapy, tumor
cavitation or proximity to major blood vessels. Eligible patients
could have either squamous or non-squamous histology. The
studies were conducted according to the Declaration of Helsinki.
Ethics committee approval and informed patient consent were
obtained before the start of the trials. The trial was registered on
ClinicalTrials.gov: NCT00832494. Study subjects received carboplatin (area under the curve
[AUC] = 6 mg/mL•min), paclitaxel (175 mg/m2), and ASA404
(1200 mg/m2 or 1800 mg/m2) (CP + ASA404) or CP alone.
For the purpose of this retrospective review, phase II data for
activity and safety were pooled by histology and by treatment,
with aggregation of the two ASA404 doses. Treatment-emergent adverse events (AEs) of grade ≥3 were defined according to the
National Cancer Institute Common Terminology Criteria for
Adverse Events (NCI-CTCAE V.3). Safety and activity results
were compared between groups of patients with squamous and
non-squamous histology: (1) receiving the same treatment; and
(2) receiving CP + ASA404 or CP alone. Treatment differences
between groups were assessed by calculating the percentage
difference (for response rates) and hazard ratio (for time-toevent
endpoints) with the corresponding 95% confidence
interval (CI). Differences in safety responses were calculated
using Fisher’s exact test. Statistically significant differences are
indicated by P<0.05.
|
|
Results
A total of 108 patients were recruited, of whom 104 were
included in the safety population (CP + ASA404, n=68; CP,
n=36), and 100 were evaluable for activity (CP + ASA404, n=64;
CP, n=36). Details on patients excluded from the analysis are
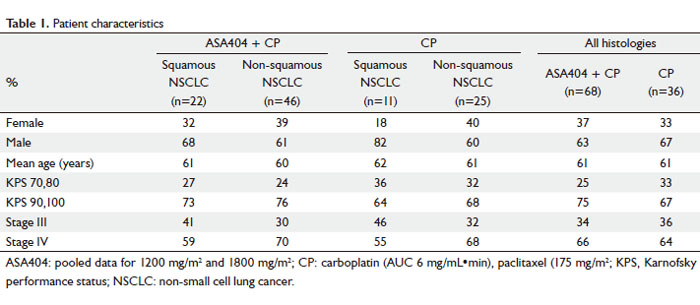
published elsewhere ( 9, 10). Characteristics of patients included
in this analysis are shown in Table 1. The treatment groups contained similar proportions of
patients with squamous and non-squamous histology. Squamous
histology was present in 31% of patients treated with CP alone
and 32% of patients treated with CP + ASA404 in the pooled
safety population, and in 31% of patients treated with CP alone
and 33% of patients treated with CP + ASA404 in the pooled
activity population ( 9, 10).
Safety
Addition of ASA404 to standard doses of CP was generally well
tolerated in patients with both squamous and non-squamous
histology. There were no AEs of NCI-CTCAE grade ≥3
associated with the vascular effects of bleeding, pulmonary
hemorrhage, hemoptysis, hypertension or proteinuria in patients
(all histologies) treated with CP + ASA404.
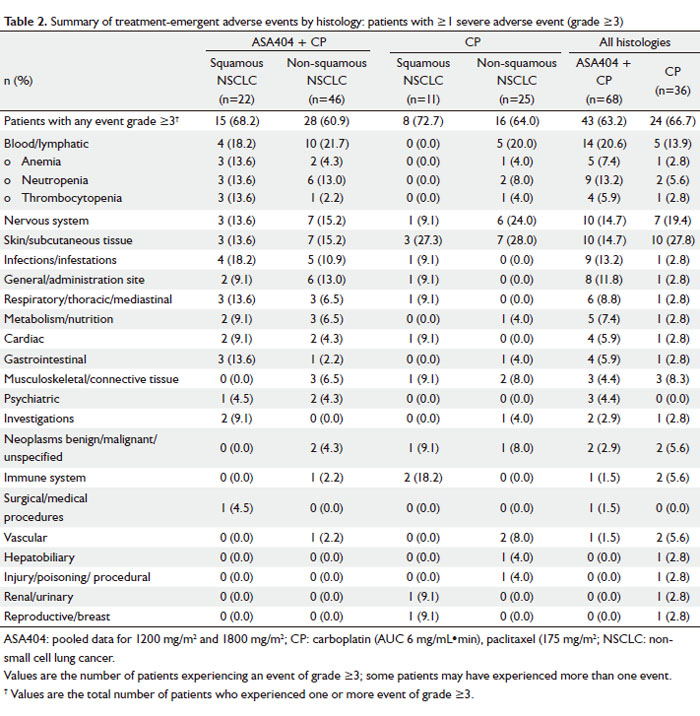
In both histologic groups, blood and lymphatic disorders were
the most frequently reported grade ≥3 AEs ( Table 2). There was
no significant difference in the proportion of patients receiving
CP + ASA404 who experienced grade ≥3 anemia (13.6% vs
4.3%; P=0.32), neutropenia (13.6% vs 13.0%; P=1.00), and
thrombocytopenia (13.6% vs 2.2%; P=0.10) in those with
squamous compared with non-squamous histology, respectively.
There were also no significant differences in the rates of grade
3/4 anemia, neutropenia or thrombocytopenia in patients
with squamous vs non-squamous histology receiving CP alone
(P=1.00 for each comparison). Comparison by treatment (all
histologies) showed rates of grade 3/4 blood and lymphatic AEs
of 13.9% and 20.6% (P=0.59) for CP alone and CP + ASA404,
respectively. Similarly, rates of individual blood and lymphatic
AEs were not statistically different when ASA404 was added to CP: grade 3/4 anemia (2.8% and 7.4%; P=0.66), neutropenia
(5.6% and 13.2%; P=0.32), and thrombocytopenia (2.8% and
5.9%; P=0.66) for CP alone and CP + ASA404, respectively.
In patients with squamous histology, CP + ASA404 resulted in
three reports (13.6%) each of grade 3/4 anemia, neutropenia
and thrombocytopenia, which was not statistically different from
the rates reported in patients treated with CP alone (P=0.53).
The non-squamous subgroup also exhibited similar rates of
grade 3/4 anemia (4.0% and 4.3%; P=1.00), neutropenia (8.0%
and 13.0%; P=0.70), and thrombocytopenia (4.0% and 2.2%;
P=1.00) for CP alone and CP + ASA404, respectively. Five cardiac events of grade ≥3 were reported: two patients
with squamous NSCLC receiving ASA404 1200 mg/m2 (angina
pectoris, tachyarrhythmia), two patients with non-squamous
NSCLC receiving ASA404 1200 mg/m2 (cardiomyopathy,
myocardial ischemia), and one patient with squamous NSCLC
receiving CP alone (tachycardia). No cardiac AEs occurred in
the ASA404 1800 mg/m2 dose cohort.
Anti-tumor activity
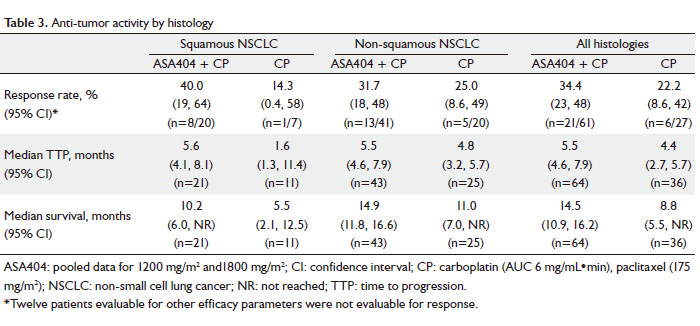
In patients with squamous histology, median survival was
10.2 months (95% CI: 6.0–NR [not reached]) for patients
receiving CP + ASA404 compared with 5.5 months (95% CI: 2.1
–12.5) for CP alone. In patients with non-squamous histology,
median survival was 14.9 months (95% CI: 11.8–16.6) for
patients receiving CP + ASA404 compared with 11.0 months
(95% CI: 7.0–NR) for CP alone. Regardless of histology, the
pooled median survival was 14.5 months (95% CI: 10.9–16.2)
for patients receiving CP + ASA404 compared with 8.8 months
(95% CI: 5.5–NR) for CP alone. RECIST response outcomes,
TTP and median survival are shown in Table 3.
|
|
Discussion
In this retrospective, pooled analysis of a phase II, multicentre,
open-label study ( 9), and single-arm extension study ( 10), the
safety and activity of ASA404 in combination with standard CP
chemotherapy were evaluated in patients with squamous and
non-squamous stage IIIb/IV NSCLC. This analysis was limited
by its retrospective nature, and by the small size of the overall
group (n=104), treatment, and disease subgroups. Although
strong conclusions cannot be made, these findings inform the
design of definitive phase III studies of ASA404 by supporting
inclusion of both squamous and non-squamous NSCLC
patients. In combination with CP, ASA404 was well tolerated in
advanced NSCLC patients regardless of squamous or nonsquamous
histology. The profile of treatment-emergent
AEs reported with ASA404 was similar to those typically
associated with standard therapy. Although the incidence of
thrombocytopenia and anemia was slightly higher in patients
with squamous histology, it was generally manageable. The
incidence of cardiac AEs was numerically higher in patients of all
histologies receiving the ASA404 combination compared with
CP alone (4 vs 1 patient). However, a casual relationship was not
established to ASA404 as these events occurred in patients with
pre-existing cardiovascular disorders. Cardiac safety of ASA404
should continue to be monitored in future studies.
This study was not powered for a statistical comparison
of activity outcomes; however, the combination of CP and
ASA404 showed a trend towards improved response rate, TTP
and median survival in patients with both squamous and nonsquamous
NSCLC compared with those receiving CP alone.
Notably, in patients with squamous histology, the addition
of ASA404 to chemotherapy resulted in an improvement in median survival vs chemotherapy alone (10.2 vs 5.5 months,
respectively). However, interpretation of these data is limited by
the retrospective nature of the analysis and the small sample size.
Currently, first-line treatment of squamous NSCLC consists of
standard chemotherapy-based regimens. New targeted therapies
and chemotherapeutic agents have been evaluated in NSCLC,
but many show little promise as first-line treatments in patients
with squamous histology ( 4-7). For example, overall survival was
less favorable with first-line pemetrexed plus cisplatin than with
gemcitabine plus cisplatin in patients with squamous NSCLC
(9.4 months vs 10.8 months, respectively; P=0.05) ( 4). In light of these findings, the use of pemetrexed is now limited to patients
with non-squamous histology ( 4). Moreover, in a phase III trial
of the multiple tyrosine kinase inhibitor (TKI) sorafenib in
combination with CP, mortality rates in patients with squamous
NSCLC receiving the sorafenib combination were higher than
in those receiving CP alone ( 7). Similarly, in combination with
CP, the TKI-based vascular endothelial growth factor inhibitor
motesanib increased mortality over standard chemotherapy
in patients with squamous NSCLC ( 5). This phase III study,
MONET-1, was suspended by the Data Safety Monitoring
Board, although it has recently been reopened for patients with non-squamous NSCLC only ( 5). The anti-angiogenic agent, bevacizumab, was evaluated in a
randomized phase II study in combination with standard CP
chemotherapy in previously untreated patients with locally
advanced or metastatic NSCLC. Six major life-threatening
pulmonary hemorrhages occurred in patients receiving the
bevacizumab-containing regimen ( 6). This outcome was
more common in patients with squamous histology (4 of
13 patients, 30.8%) than in those with non-squamous histology
(2 of 54 patients, 3.7%), and may be explained by the fact that
squamous tumors are often central, large and grow in close
proximity to major blood vessels. Despite these findings being
described in only a small number of patients, this early signal
resulted in limitation of the phase III clinical development
program and subsequent registration of bevacizumab in NSCLC
to patients with non-squamous histology only ( 12). In the
present study, despite almost one-third of patients having
squamous histology, no cases of major pulmonary hemorrhage
were reported in patients treated with ASA404 in combination
with CP. An apparent lack of severe adverse vascular effects
may be unexpected for a drug causing tumor hemorrhagic
necrosis, but could be attributed to the distinct anti-vascular
action of ASA404 compared with anti-angiogenic agents such as
bevacizumab.
|
|
Conclusions
Phase II evaluations suggest that ASA404 is a promising
addition to standard chemotherapy for the first-line treatment of
NSCLC, regardless of histology. This small analysis indicates that
ASA404 has a similar safety and activity profile in patients with
squamous and non-squamous NSCLC but this finding must be confirmed by larger prospective studies. The phase III study
of ASA404 as a first-line treatment for NSCLC in combination
with chemotherapy (ATTRACT-1) has been halted following
interim data analysis showing futility ( 13). However, no safety
concerns were identified and the phase III second-line study
in combination with docetaxel (ATTRACT-2) is ongoing. The
latter phase III study includes patients with both squamous and
non-squamous histologies. The current retrospective analysis
of pooled data from two small phase II studies has several
limitations but informs the design of these subsequent larger
definitive trials.
|
|
Acknowledgements
We thank the patients, families, study staff, co-investigators
and the Antisoma clinical development team. The work was
supported by funding from Antisoma Research Limited, London,
UK. Editorial assistance was provided by Articulate Science,
London, UK on behalf of Novartis Pharmaceuticals Corporation.
Dr Mark McKeage has received funding for research, together
with consulting and speaker fees from Antisoma and Novartis.
Dr Michael Jameson has no competing interests to disclose.
Dr Mark McKeage and Dr Michael Jameson contributed to
the conception of the study, its design, coordination, patient
recruitment and data analysis and interpretation. Both authors
participated in drafting the manuscript, and read and approved
the final version.
|
|
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin 2008;58:71-96.[LinkOut]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80.[LinkOut]
- Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer 1995;75:191-202.[LinkOut]
- Scagliotti GV, Parikh P, Von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage nonsmall- cell lung cancer. J Clin Oncol 2008;26:3543-51.[LinkOut]
- Chustecka Z. Phase 3 trial of motesanib in non-small-cell lung cancer suspended. Available from: [http://www.medscape.com/viewarticle/584015.[LinkOut]
- Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small cell lung cancer. J Clin Oncol 2004;22:2184-91.[LinkOut]
- Scagliotti GV, Novello S, Von Pawel J, Reck M, Pereira J, Thomas M, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:1835-42.[LinkOut]
- Baguley BC. Antivascular therapy of cancer: DMXAA. Lancet Oncol 2003;4:141-8.[LinkOut]
- McKeage MJ, Von Pawel J, Reck M, Jameson MB, Rosenthal MA, Sullivan R, et al. Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br J Cancer 2008;99:2006-12.[LinkOut]
- McKeage MJ, Reck M, Jameson MB, Rosenthal MA, Gibbs D, Mainwaring PN, et al. Phase II study of ASA404 (vadimezan, 5,6-dimethylxanthenone- 4-acetic acid/DMXAA) 1800mg/m2 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Lung Cancer 2009;65:192-7.[LinkOut]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16.[LinkOut]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50.[LinkOut]
- ATTRACT-1 phase III trial of ASA404 halted following interim
analysis. Available from: http://www.antisoma.com/asm/media/press/
pr2010/2010-03-29/.[LinkOut]
Cite this article as: McKeage MJ, Jameson MB, AS1404-201 Study Group Investigators. Comparative outcomes of squamous and non-squamous non-small cell lung cancer (NSCLC) patients in phase II studies of ASA404 (DMXAA) – retrospective analysis of pooled data. J Thorac Dis 2010; 2(4): 199-204. doi: 10.3978/j.issn.2072-1439.2010.02.04.1
|





