Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis
Introduction
Lung cancer is still the leading cause of cancer death globally. Non-small cell lung cancer (NSCLC) accounts for 85–90% of all cases (1). Almost half of patients diagnosed with NSCLC have distant metastases at presentation (2) and the prognosis of these patients are poor. Systemic therapy is considered to be the standard treatment for stage IV NSCLC, which results in median survivals of 8–11 months (3). Targeted therapy have shown efficacy in the treatment of advanced NSCLC, however, most patients progress after a median survival of 12 months because of resistance (4-6).
Hellman and Weichselbaum proposed the existence of a clinically recognisable state termed ‘oligometastases’, which is an intermediate state of metastases characterized by a limited number and site of metastatic tumors. In these selected patients, eradication of oligometastases could be curative (7,8). However, whether the aggressive thoracic therapy (ATT) could potentially improve overall survival (OS) in oligometastatic NSCLC remains unclear. Removal of the primary tumor was shown to improve OS in patients with metastatic renal cancer (9), by contrast, locoregional treatment of the primary tumor did not affect OS in patients with metastatic breast cancer (10). More and more studies reported favorable long-term survivals in oligometastatic NSCLC treated with aggressive local therapy (11-13). However, these studies are retrospective and the validity of the survival benefit are challenging due to patient selection.
Recently, Ashworth et al. did a systematic review of literature considering NSCLC patients with 1–5 metastases treated with local therapy and found that definitive treatment of the primary tumor was a good prognostic factor for survival (14). Moreover, they further did an individual patient data (IPD) meta-analysis and found that long-term survival is common in selected patients with metachronous oligometastases, while patients with synchronous metastases and N1/N2 disease experienced the poorest survival (15). In light of these findings, we performed this meta-analysis to determine whether synchronous oligometastatic NSCLC patients might benefit from ATT, and to assess the long-term survival of patients with synchronous oligometastatic NSCLC who received or did not receive ATT .
Methods
Search strategies
A comprehensive systematic search for published articles was carried out by two authors independently up to Aug 3, 2015 among the PubMed, EMBASE, and Cochrane databases. Potential articles were identified using the following keywords combined by “non-small cell lung cancer OR non-small cell lung carcinoma OR non-small cell lung neoplasm OR non-small cell lung tumor OR NSCLC OR pulmonary adenocarcinoma OR lung adenocarcinoma OR adenocarcinoma of the lung OR lung squamous carcinoma OR pulmonary squamous carcinoma OR squamous cell lung carcinoma” AND “neoplasms/sc OR metast* OR oligomet* OR stage 4 OR stage IV OR stage four OR late stage OR advanced stage” AND “radiotherapy OR radiation therapy OR stereotactic OR SBRT OR SABR OR EBRT OR surgery OR resection OR surgical OR eradication OR ablation OR aggressive therapy” AND “primar*” AND “survive*”. We also searched the bibliography of identified articles manually.
Selection criteria
Studies meeting the following eligibility criteria were included: randomized controlled trial or retrospective or prospective observational cohort studies; studies on NSCLC patients with 1 to 5 synchronous metastases (diagnosed within 2 months after the primary tumor); studies on benefit from ATT (surgery or radiotherapy with a total dose more than 40 Gy); studies with available OS data of observed patients; and studies in English. The exclusion criteria were as followings: review papers, case reports. and non-controlled clinical studies;. studies that only reported the therapy of metastases; studies with fewer than 20 patients.
As regard to studies with overlapping patient populations, the most recent investigation using updated data and with the maximum number of individuals was chosen for inclusion.
Data extraction
Data were extracted and recorded in a predefined information sheet by two authors independently, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (16). Study characteristics (first author, country of authors, time period of enrolling patients, number of patients, oligometastatic state, ATT regimens) and outcome data were extracted from the included articles. All discrepancies were discussed to reach consensus by including a third authors. The assessment of the risk of bias in included studies was conducted independently by two authors using Newcastle-Ottawa scale (17).
Statistical analyses
We predefined OS as the primary outcome and a hazard ratio (HR) expressing the intervention effect. For each study, the HR with corresponding 95% confidence intervals (CI) was directly extracted from the research article or estimated using Kaplan-Meier survival curves (18).
The χ2 test and I2 test were used to determine the heterogeneity (19). If the analysis showed significant heterogeneity, defined as Pheterogeneity<0.1 or I2>30%, the random model was used. Otherwise, the fixed model was used. All statistical analyses were performed using the Review Manager analysis software (RevMan, version 5.1.2: The Cochrane Collaboration, http://ims.cochrane.org/revman). A P value of less than 0.05 was considered to be statistically significant. Successive monthly pooled estimates of survival and its standard errors were calculated and then used to yield a pooled measure of cumulative OS for each intervention group, according to the methods suggested by Pereira et al. and Takagi et al. (20,21). Statistical significance (P<0.05) was assessed for the cumulative survival rate differences between ATT group and no-ATT group at yearly intervals. The statistical method used judges the significance of differences by examining the overlap between confidence intervals, but it does not calculate P values (22).
Results
Study characteristics
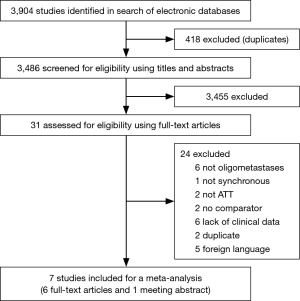
A total of 3,904 articles were retrieved from databases search. According to the eligibility criteria for title and abstract, 31 articles were selected for full-text screening, In total, 6 full-text articles (23-28) and 1 meeting abstract (29) were selected for the final analysis. A flow diagram depicting literature screening process is shown in Figure 1.
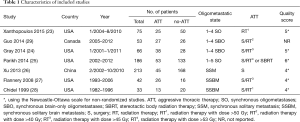
The characteristics of the 7 included studies are summarized in Table 1. All the included studies are retrospective observational cohort studies from 1999 to 2015, consisting of 668 synchronous oligometastatic NSCLC patients, among whom 227 (34.0%) received ATT. With regard to ATT regimens, Surgery only was the modality of ATT in one study (26), radiotherapy in one study (23), and surgery combined with radiotherapy or not in the five others articles (24,25,27-29). When stratifying by oligometastatic state, single organ metastases was reported in five studies (24,26-29), of which four included only patients with brain-only metastases (24,27-29).
Full table
Benefit of ATT for OS
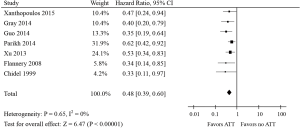
Incorporating ATT into the treatment of patients with synchronous oligometastatic NSCLC was statistically significantly associated with a 52% reduction in the risk of death (HR, 0.48; 95% CI, 0.39–0.60; P<0.00001; Figure 2). No significant statistical heterogeneity was noted for this outcome (I2=0%, P=0.65). No test for funnel plot asymmetry was used, because the power of the tests was too low to distinguish chance from real asymmetry when there were only seven studies included in this meta-analysis (30).
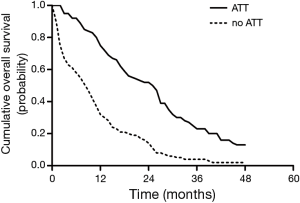
Data for successive monthly pooled estimates of survival were available from 5 studies (23,24,26-28), consisting of 429 patients, and of whom 155 patients received ATT. The pooled survival curves of OS were displayed graphically as Figure 3. The pooled cumulative survival rates for patients received ATT were 74.9% (95% CI, 62.7–87.1%) at 1 year, 52.1% (95% CI, 40.3–64.0%) at 2 years, 23.0% (95% CI, 10.6–35.5%) at 3 years, and 12.6% (95% CI, 3.6–21.6%) at 4 years. The corresponding pooled OS for patients who did not receive ATT were 32.3% (95% CI, 20.8–43.7%), 13.7% (95% CI, 6.7–20.7%), 3.7% (95% CI, 0–8.0%), and 2.0% (95% CI, 0–4.7%), respectively. The 1-, 2-, 3-year cumulative survival rate differences between ATT group and no-ATT group were statistically significant.
Subgroup analyses
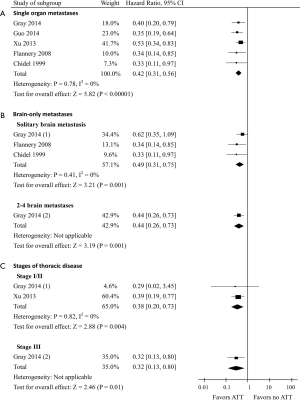
In the subgroup with single organ metastases (data of 407 patients was available), the pooled HR for OS was 0.42 (95% CI, 0.31–0.56; P<0.00001; Figure 4A). Among the brain-only metastases subgroup, the pooled HR for OS in patients presenting solitary brain metastasis (n=108) was 0.49 (95% CI, 0.31–0.75) compared with a HR of 0.44 (95% CI, 0.26–0.73) in patients presenting 2-4 brain metastases (n=33) (Figure 4B). Furthermore, the HR for OS was 0.38 (95% CI, 0.20–0.73; P=0.004) and 0.32 (95% CI, 0.13–0.80; P=0.01) in patients with thoracic stage I–II disease (data of 96 patients was available) and patients with thoracic stage III disease (data of 47 patients was available), respectively (Figure 4C).
Discussion
To our knowledge, this is the first meta-analysis to explore the survival benefit of ATT in synchronous oligometastatic NSCLC patients. Using data from 7 retrospective cohort studies including 668 synchronous oligometastatic NSCLC patients, we demonstrated that treatment with ATT is associated with a 52% overall reduction in the risk of death. Pooled 1-, 2-, 3- and 4-year survival for those receiving ATT were 74.9%, 52.1%, 23.0%, and 12.6% respectively vs. 32.3%, 13.7%, 3.7%, and 2.0% for those receiving no-ATT.
The definition of oligometastatic state is not uniform and studies included patients with limited number of metastatic foci, varying from 1 to 5. In the other hand, patients with innumerable metastatic lesions and then responded well enough to have a more finite number of lesions after systemic therapy- with no limitation to any duration of disease control will be considered to be with oligometastatic foci as well, leading the term “limited” to be vague (31). In all, we included studies on NSCLC patients with 1 to 5 synchronous metastases at first presentation without treatment.
Brain metastases occur in 30–50% of NSCLC patients and generally have a poor prognosis (32,33). For synchronous brain metastases, a recent study showed that patients with multiple metastases who received ATT had significantly improved survival vs. no-ATT, and this trend also was observed in those with a solitary metastasis (24). However, recent European guidelines did not recommend ATT for patients with more than one brain metastasis (34). In our subgroup analysis, aggressive management of thoracic disease improves the survival in NSCLC patients either with solitary brain metastasis or with 2-4 brain metastases. Our subgroup analysis also showed that single organ oligometastatic patients with ATT had a better OS. Due to the lack of survival data detailed by other metastatic organs in present studies, any firm conclusion cannot be drawn yet.
Although the OS benefit from ATT was verified in patients with synchronous oligometastatic NSCLC, it is still unclear who of these patients may benefit more from ATT. Factors affecting survival of synchronous oligometastatic NSCLC patients, such as performance status, N-stage, and thoracic stage, have been revealed in some reports (35-37). However, no study investigated factors predictive of survival specially for patients with ATT. In this meta-analysis, we performed stratified analysis according to intrathoracic disease stage, and concluded that patients of all thoracic stages could benefit from ATT.
It’s been reported that primary or metastatic tumors within lung or liver treated to a nominal dose of 54 Gy or greater had a 3-year actuarial local control rate of 89.3% compared with 59.0% and 8.1% for those treated to 36–53.9 Gy and less than 36 Gy (38), which indicated that radiotherapy dose-dependently improved the local control of primary or metastatic tumors. Guerra et al. recently found that receipt of at least 63 Gy to the primary tumor could be delivered safely and was an independent prognostic predictor of favorable outcomes in patients with oligometastatic NSCLC at diagnosis (39). These results implied that improved local control achieved by radiotherapy could be transformed into long-term survival benefit. However, in the included studies, ATT was achieved with radiotherapy only to a dose of more than 40 Gy, which might result in an underestimation of the actuarial survival benefit from ATT. What is more, no study evaluates the association between different therapeutic approaches used in ATT with OS, such as surgery vs. radiotherapy.
The gold-standard for synthesising survival data from clinical trials is to obtain IPD from each study (40). However, IPD was not available in our study and we pooled survival curves basing on estimated data alternatively. The pooled survival curves also suggested an OS benefit from ATT over no-ATT in patients with synchronous oligometastatic NSCLC. Limitations of this method were still existed, such as omitted losses to follow-up, a flat tail in the survival curve (20) and so on.
Recently, a phase 2 randomized study found that local consolidative therapy with or without maintenance therapy for patients with three or fewer metastases from NSCLC that did not progress after initial systemic therapy improved progression-free survival compared with maintenance therapy alone (41). This study support our findings at some degree, however, we focus aggressive therapy particularly to the primary tumor and patients without treatment before. In general, randomized controlled trials are still warranted to further support our findings.
There are several limitations of the present meta-analysis. First, all the 7 included studies were retrospective cohort studies and there was no randomized controlled trial or prospective cohort study. Second, patients included in our study were selected and especially contains a significantly higher proportion of patients with single brain metastases than is seen in actual clinical practice. Third, being limited by language, we only included studies published in English, and 5 potentially eligible studies were excluded because of language, 2 in German, 2 in Japanese, and 1 in Italian. Additionally, given EGFR and ALK mutation status unknown for the bulk of the included patients in our study, it remains unclear whether there would be a similar finding in the setting of EGFR or ALK TKIs, such as gefitinib, erlotinib or afatinib, as systemic therapy.
Conclusions
The meta-analysis indicated that ATT may improve OS in patients with synchronous oligometastatic NSCLC. The relative OS benefits of ATT were also seen in patients with brain metastases and patients of all thoracic stages. These findings have important implications for clinical trial design and intervention, however, randomized controlled trials are still warranted to further support our results. Moreover, we suggest further studies to optimize treatment regimen of ATT, and to identify clinical or molecular predictors for the selection of patients who will benefit more from ATT.
Acknowledgements
Funding: This study was funded by National Natural Science Foundation of China (81572279, 81001047), University Excellent Young Teachers Program of Guangdong Province (Yq2013040), Natural Science Foundation of Guangdong Province (2015A030313253), and Pearl River Nova Program of Guangzhou City (2014J2200031).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551-64. [Crossref] [PubMed]
- Grossi F, Kubota K, Cappuzzo F, et al. Future scenarios for the treatment of advanced non-small cell lung cancer: focus on taxane-containing regimens. Oncologist 2010;15:1102-12. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Jänne PA, Wang X, Socinski MA, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol 2012;30:2063-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [Crossref] [PubMed]
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380-8. [Crossref] [PubMed]
- Endo C, Hasumi T, Matsumura Y, et al. A prospective study of surgical procedures for patients with oligometastatic non-small cell lung cancer. Ann Thorac Surg 2014;98:258-64. [Crossref] [PubMed]
- Yano T, Okamoto T, Haro A, et al. Local treatment of oligometastatic recurrence in patients with resected non-small cell lung cancer. Lung Cancer 2013;82:431-5. [Crossref] [PubMed]
- Villarreal-Garza C, de la Mata D, Zavala DG, et al. Aggressive treatment of primary tumor in patients with non-small-cell lung cancer and exclusively brain metastases. Clin Lung Cancer 2013;14:6-13. [Crossref] [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale(NOS) for assessing the quality of nonrandomised studies inmeta-analyses. Available online: [Accessed October 6, 2014].http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Pereira CE, Albers M, Romiti M, et al. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg 2006;44:510-7. [Crossref] [PubMed]
- Takagi H, Umemoto T. ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. A meta-analysis pooling survival curves in randomized controlled trials and propensity-score matched studies of endovascular versus open abdominal aortic aneurysm repair. Int J Cardiol 2014;174:785-8. [Crossref] [PubMed]
- Schenker N, Gentleman JF. On Judging the Significance of Differences by Examining the Overlap Between Confidence Intervals. The American Statistician 2001;55:182-6. [Crossref]
- Xanthopoulos EP, Handorf E, Simone CB 2nd, et al. Definitive dose thoracic radiation therapy in oligometastatic non-small cell lung cancer: A hypothesis-generating study. Pract Radiat Oncol 2015;5:e355-63. [Crossref] [PubMed]
- Gray PJ, Mak RH, Yeap BY, et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014;85:239-44. [Crossref] [PubMed]
- Parikh RB, Cronin AM, Kozono DE, et al. Definitive primary therapy in patients presenting with oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;89:880-7. [Crossref] [PubMed]
- Xu Q, Wang Y, Liu H, et al. Treatment outcome for patients with primary NSCLC and synchronous solitary metastasis. Clin Transl Oncol 2013;15:802-9. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Regine WF, et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys 2008;72:19-23. [Crossref] [PubMed]
- Chidel MA, Suh JH, Greskovich JF, et al. Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat Oncol Investig 1999;7:313-9. [Crossref] [PubMed]
- Guo G, Lambert P, Ahmed N, et al. Local Treatment Improves Survival in NSCLC Patients With Synchronous Brain Oligometastases. Int J Radiat Oncol Biol Phys 2014;90:S52. [Crossref]
- Song F, Eastwood AJ, Gilbody S, et al. Publication and related biases. Health Technol Assess 2000;4:1-115. [PubMed]
- West HJ. The Slippery Slope of Broadening Treatment Eligibility and Weak End Points: Defending the Oligo in Oligometastatic Non-Small-Cell Lung Cancer. JAMA Oncol 2015;1:1219-20. [Crossref] [PubMed]
- Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80. [Crossref] [PubMed]
- Rodrigus P, de Brouwer P, Raaymakers E. Brain metastases and non-small cell lung cancer. Prognostic factors and correlation with survival after irradiation. Lung Cancer 2001;32:129-36. [Crossref] [PubMed]
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii56-64. [Crossref] [PubMed]
- Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [Crossref] [PubMed]
- Kanou T, Okami J, Tokunaga T, et al. Prognosis associated with surgery for non-small cell lung cancer and synchronous brain metastasis. Surg Today 2014;44:1321-7. [Crossref] [PubMed]
- Iwasaki A, Shirakusa T, Yoshinaga Y, et al. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg 2004;26:488-93. [Crossref] [PubMed]
- McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2009;73:112-8. [Crossref] [PubMed]
- Lopez Guerra JL, Gomez D, Zhuang Y, et al. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int J Radiat Oncol Biol Phys 2012;84:e61-7. [Crossref] [PubMed]
- Arends LR, Hunink MG, Stijnen T. Meta-analysis of summary survival curve data. Stat Med 2008;27:4381-96. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]