CT-guided transthoracic core needle biopsy for small pulmonary lesions: diagnostic performance and adequacy for molecular testing
Introduction
Making a histological diagnosis for potential malignant pulmonary lesions relies on invasive procedures, including bronchoscopy, computed tomography (CT)-guided fine needle aspiration biopsy (FNAB), and CT-guided transthoracic needle biopsy (1,2). FNAB can only yield the cytological features of lesions rather than the histopathological results of lesions. It has been reported that the diagnostic accuracy of FNAB is decreased for small lesions (3,4). CT-guided transthoracic needle biopsy is a safe technology that is easy to operate, with a diagnostic accuracy for pulmonary lesions that reaches 64–97% (5-8). Therefore, this procedure has become one of the major methods for determining the nature of pulmonary lesions.
In previous studies, 20- to 22-gauge needles were commonly used for transthoracic needle biopsy because of their good diagnostic yield and low complication rate (9-17). However, in the era of precision medicine, relying only histopathological diagnosis and subtyping of lung tumors by immunohistochemistry analysis is insufficient for making optimal treatment decisions. Molecular analysis of driver mutations is strongly recommended for the precise treatment of advanced non-small cell lung cancer (NSCLC) (18). Until now, few large studies have evaluated the diagnostic performance and complications of transthoracic core needle biopsy (TCNB) using an 18-gauge cutting needle, which is favorable to acquire samples in larger volume than 20- or 22-gauge needle. The suitability of specimens obtained by TCNB for driver mutation testing is uncertain. Furthermore, the recommended number of samples to take from a lesion for molecular analysis was not reported previously.
To determine the clinical value of TCNB for small pulmonary lesions, we conducted a study on the diagnostic outcomes and complication rates of CT-guided TCNB using an 18-gauge cutting needle for small (≤3 cm) pulmonary lesions in 560 consecutive patients. We assessed the risk factors of diagnostic failure using univariate and multivariate analyses. Additionally, we reported the adequacy of TCNB for obtaining material sufficient for molecular testing.
Methods
This retrospective study was approved by the institutional review board of the hospital (Approval number: 2016-85). The study outcomes will not affect future patients management.
Study population
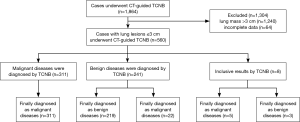
Patients were enrolled if they underwent CT-guided TCNB in the department of respiratory and critical care medicine at our tertiary referral center between January 2012 and January 2015. During this period, 1,864 consecutive cases underwent CT-guided TCNB due to a clinical suspicion of pulmonary malignancy. All biopsies were performed for the diagnostic results of transbronchial examination were negative. The patients expected to obtain a definite diagnosis. The procedure was performed after informed consent was obtained, although the lesions less than 1 cm especially in basal regions of the lung are very difficult to biopsy.
Among them, 1,240 cases had lung masses >3 cm, and 624 cases had lung lesions ≤3 cm (Figure 1). Excluding 64 cases with incomplete data or for whom it was impossible to obtain follow-up information, 560 total biopsy cases of small pulmonary lesions were included in the analysis. Among the pulmonary lesion cases, 61 were ≤1 cm, 214 were 1.1–2.0 cm, and 285 were 2.1-3.0 cm. The patient ages were 51.8±12.7 (range, 11–83) years, among which 323 were male (aged 11–83 years), and 237 were female (aged 15–82 years). There were 453 cases of solid pulmonary lesions, 34 cases of ground-glass nodules, and 73 cases of mixed nodules. The mean size of the 560 pulmonary lesions was 1.8±0.6 cm in diameter (range, 0.6–3.0 cm).
Biopsy procedures
All biopsies were performed by two experienced physicians (PWT and YW, with 9 years of experience in respiratory medicine and 4 years of experience with CT-guided needle biopsy, respectively). The procedure was conducted under CT guidance (SOMATOM Definition AS+ 64, Siemens, Germany). The biopsy system consisted of a 17-gauge coaxial introducer and an 18-gauge automated cutting needle (BARD, New Jersey, US). To design the shortest route, the patients underwent CT scans in a supine, prone, or lateral position. Images were obtained from the region of interest using a 4-mm section thickness. The chosen entry site was prepared in a sterile fashion. After local anesthesia with 2% lidocaine, the coaxial introducer was inserted along the designed path. A CT scan was conducted again to confirm that the coaxial introducer was placed at the target lesion. The cutting biopsy needle was inserted into the target lesion through the coaxial introducer, and the biopsy was then performed (Figure 2). Specimens were repeatedly obtained until adequate material had been collected for pathological examination. Two or three passes were taken from each lesion for most cases. Because of objective conditions, we did not use care vision during biopsy.

The specimens were fixed in 10% formalin and were then immediately transported to the pathology department. After the procedure, the patient underwent CT scan to exclude pneumothorax or bleeding due to considerations of safety, although it was not obligatory. For the next 24 hours, the patients were monitored for potential complications in a ward. The patients were observed for cough, hemoptysis, shortness of breath, chest tightness, and other symptoms, and records were taken.
Diagnostic criteria
The final diagnosis was confirmed in one of three ways: (I) if a patient underwent surgical resection, the final diagnosis was determined based on the pathology reports of the resected specimens; (II) if the TCNB or other biopsy methods revealed malignancies or specific benign abnormalities (e.g., mycosis or tuberculosis), the diagnosis was determined based on a combination of pathology reports, laboratory findings, and the clinical course; (III) if non-specific benign abnormality was indicated in a pathology report, the lesion was considered benign if it regressed with conservative medical treatment or remained stable. The mean duration of follow-up was 1 year. Pathologic results were considered inconclusive if malignancies were suspected but not confirmed.
Positive biopsy results were further categorized as true positive or false positive if the final diagnosis was malignant or benign abnormalities, respectively. Likewise, negative biopsy results were further categorized as true negative or false negative if the final diagnosis was benign or malignant abnormalities, respectively. We divided all biopsy cases into three groups, a ≤1-cm group, 1.1–2.0-cm group, and 2.1–3.0-cm group, and calculated the above data and diagnostic accuracy of each group. We also calculated the overall sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of diagnosis of malignant diseases by TCNB.
Adequacy for molecular testing of TCNB specimens
The molecular testing data for driver gene mutations of lung cancer were collected. The molecular testing protocol for lung cancer varied over time as new testing targets and methods became available (19). Molecular testing was prompted on cases with lung adenocarcinoma or NSCLC. Initially, the reflexive testing was epidermal growth factor receptor (EGFR) mutation analysis and it was then expanded to include anaplastic lymphoma kinase (ALK) translocation by fluorescence in situ hybridization (FISH). EGFR mutation analysis was performed with polymerase chain reaction using the amplification-refractory mutation system (ARMS). For ARMS-based EGFR testing, at least 200 tumor cells on formalin-fixed, paraffin-embedded (FFPE) specimens were accepted for analysis. ALK detection was done on tumor tissues using an LSI ALK Dual Color Break Apart Rearrangement Probe (Abbott-Vysis, Downers Grove, Ill) per the manufacturer’s instructions. A minimum of 100 tumor cells should be counted for the interpretation of each case. Successful molecular analysis was defined as the capacity to yield the presence or absence of EGFR mutation and ALK rearrangement.
Statistical analysis
The characteristics of the patients, lung lesions, and biopsy procedures were collected for statistical analysis. All patients were divided into two groups: the diagnostic success group (true positive, true negative) and the diagnostic failure group (false positive, false negative, inconclusive). Different characteristics between the two groups were analyzed using Student’s t-test for numeric values and Pearson’s Chi-squared test or Fisher’s exact test for categorical values. The characteristics with P<0.05 at univariate analysis were used as input variables for multivariate logistic regression analysis to determine independent factors. An odds ratio (OR) >1 was considered an independent risk factor for diagnosis failure. If the P value was less than 0.05, it was considered a statistically significant difference. All analyses were performed using SPSS 19.0 (IBM, Chicago, IL, USA).
Results
Diagnostic performance
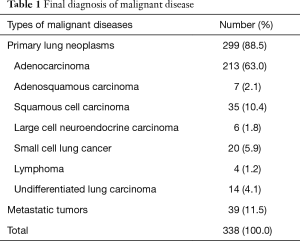
Of the 560 total pathology reports, eight (1.4%) were judged to be inconclusive and 552 (98.6%) were judged to have a definite diagnosis. In total, 311 cases were diagnosed from biopsy as malignant diseases, and they were all finally demonstrated to be true positive. Two hundred forty-one cases were diagnosed from biopsy as benign diseases; 22 of them were finally demonstrated to be malignant diseases (false negative), and the other 219 were true negatives. Eight cases were inconclusive, among which two cases (0.4%) were due to failures of technology. In the other six cases, the preliminary biopsy results revealed atypical cells, but the immunohistochemistry could not determine whether the cells were tumor cells. Three of those eight cases were finally diagnosed as benign diseases, and five were finally diagnosed as malignant diseases. In total, 338 cases were finally confirmed as malignant diseases (Table 1); 261 of them were NSCLC, including lung adenocarcinomas, adenosquamous carcinoma, squamous cell carcinoma and large cell carcinoma, and 77 were other lung neoplasms (20 small cell lung cancer, 4 lymphomas, 14 undifferentiated lung carcinoma and 39 metastatic tumors). Although the lesions were suspicious malignancy from CT, 222 cases were finally confirmed as benign diseases (tuberculosis 114 cases, non-specific inflammation 85 cases, mycosis 16 cases, Wegener’s granulomatosis 4 cases, and sclerosing hemangioma 3 cases).
Full table
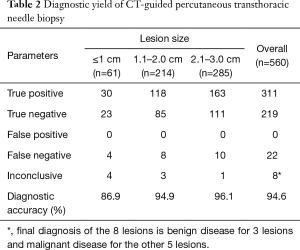
The overall sensitivity, specificity, PPV, and NPV for the diagnosis of malignancy were 92.0% (311/338), 98.6% (219/222), 100 (311/311), and 90.9% (219/241), respectively. The overall diagnostic accuracy was 94.6% (530/560). For pulmonary lesions ≤1 cm, the diagnostic accuracy was 86.9% (53/61). The diagnostic yields of CT-guided TCNB are listed in Table 2.
Full table
Complications
The most common complications were bleeding and pneumothorax; none of the patients died due to severe complications. The incidence of bleeding was 22.9% (128/560), with 117 cases of hemoptysis, including 109 cases with a small amount of hemoptysis (≤10 mL), five cases with a medium amount of hemoptysis (10–100 mL), and three cases with a large amount of hemoptysis (>100 mL). Mild hemothorax occurred in 11 cases.
The incidence of pneumothorax was 10.4% (58/560), of which 39 cases had mild pneumothorax (lung compression ≤20%), 15 cases had moderate pneumothorax (20%< lung compression ≤50%), and four cases had severe pneumothorax (lung compression >50%). These four patients recovered after thoracotomy tube placement. The patients with moderate pneumothorax improved after thoracic puncture and air aspiration, while those with mild pneumothorax recovered after self-absorption.
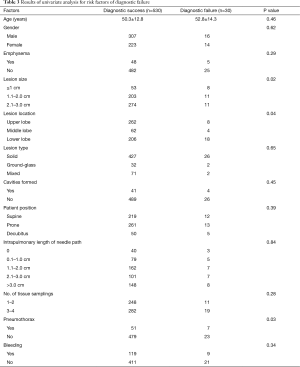
Risk factors for diagnostic failure
There were 530 cases of diagnostic success and 30 cases of diagnostic failure. The data from the univariate analysis for diagnostic failure are shown in Table 3. The risk factors demonstrating statistically significant differences between the two groups included lesion size (≤1 cm, 1.1–2.0 cm, or 2.1–3.0 cm) (P=0.02), lesion location (upper lobe, middle lobe, or lower lobe) (P=0.04), and pneumothorax (P=0.03).
Full table
The independent risk factors of logistic regression analysis in determining diagnostic failure are listed in Table 4. The independent risk factors for diagnostic failure were lesion size ≤1 cm (OR, 3.95; 95% CI, 1.67–13.96; P=0.007), lower lobe lesion (OR, 2.83; 95% CI, 1.46–5.91; P=0.001), and pneumothorax (OR, 1.98; 95% CI, 1.11–3.36; P=0.004).

Full table
Malignant diseases and molecular testing
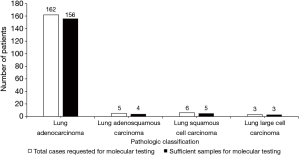
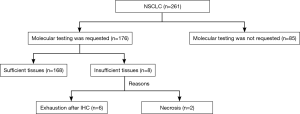
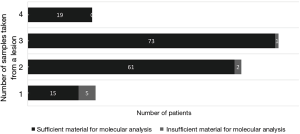
Of 261 specimens diagnosed as NSCLC, 176 were submitted for molecular testing, while the other 85 were not because of the patients’ refusal (Figure 3). For primary NSCLC including lung adenocarcinoma, adenosquamous carcinoma, squamous cell carcinoma and large cell carcinoma, sufficient tissues remained to perform molecular analysis in 95.45% of cases (168/176) (Figure 4). Only EGFR testing was performed in 102 cases, while both EGFR and ALK testing were performed in the other 66 cases. Of the 168 evaluable specimens, EGFR mutations were detected in 72 specimens (42.86%). ALK rearrangement was detected in 7.58% of cases (5/66). Eight primary lung carcinoma cases (4.55%) had insufficient tissue for molecular testing due to the exhaustion of tissue after the immunohistochemical analysis for six specimens and the presence of necrosis in two specimens. In four subgroups, in which the numbers of samples taken from one lesion were 1, 2, 3 and 4, respectively, the ratios of sufficient material for molecular analysis were 75.0% (15/20), 96.8% (61/63), 98.6% (73/74) and 100.0% (19/19), respectively (Figure 5).
Discussion
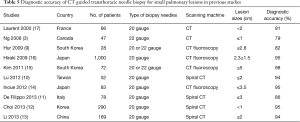
Our study showed that the overall sensitivity, specificity, and accuracy of CT-guided TCNB using an 18-gauge cutting needle were 92.0%, 98.6%, and 94.6%, respectively. The results of our study were comparable with the overall diagnostic accuracy of CT-guided biopsy with 20- to 22-gauge needles or CT fluoroscopy-guided needle biopsy (9-17). The detailed results of recent clinical studies for the diagnosis of small pulmonary lesions by CT-guided needle biopsy are shown in Table 5.
Full table
CT fluoroscopy-guided needle biopsy could be used for the diagnosis of small pulmonary lesions in basal locations, especially for uncooperative patients. Inoue et al analyzed 83 cases of lung lesions under CT fluoroscopy-guided needle biopsy using a 20-gauge cutting needle and found that the overall sensitivity, specificity, and accuracy were 95% (56/81), 100% (5/5), and 95% (63/66), respectively (14). The study of Hiraki et al. showed that for 1,000 pulmonary lesions, the sensitivity, specificity, and accuracy of CT fluoroscopy-guided needle biopsy using a 20-gauge cutting needle were 94.2% (741/787), 99.1% (211/213), and 95.2% (952/1,000), respectively (16). CT fluoroscopy allows the operating physician to continuously monitor the position of the biopsy needle during the procedure and to perform a timely adjustment based on the influences of respiratory motion (20). However, CT fluoroscopy exposes the operating physicians and patients to a large amount of radiation (21,22). Research has shown that the radiation exposure of the physicians and patients with CT fluoroscopy-guided needle biopsy is significantly greater than that with conventional CT guidance (15).
Bleeding and pneumothorax are the common complications of needle biopsy. In this study, the incidence of bleeding was 22.9%, and the incidence of pneumothorax was 10.4%. Two previous studies of needle biopsy using a 20-gauge cutting needle reported that the rates of bleeding were 17.8% and 19.3%, respectively (13,17), which are lower than the results of our study. Previous studies have shown that the biopsy-induced pneumothorax rate ranged from 15.0% to 35.0% (23-26). Compared with the above studies, the incidence of pneumothorax in our study was lower. The pneumothorax incidence in this study was confirmed by precise imaging findings. The patients who experienced shortness of breath after the procedure were excluded from the statistics if the imaging examination was not completed.
A lesion diameter ≤1 cm is an independent risk factor for biopsy diagnostic failure. This result of the present study is consistent with the results reported by Hiraki et al. (16). Typically, the placement of a needle coaxially in pulmonary lesions with diameters ≤1 cm presents greater technical difficulties.
A lower lobe lesion is another independent risk factor for biopsy diagnostic failure, as also shown by Lee et al. (27). Lesions in basal regions of the lung are very difficult to biopsy because of the influence of respiratory motion. The coaxial needle must be adjusted to achieve the target site, which will increase the damage to normal lung tissue. The impact of respiratory motion on the upper and middle lobes is mild; therefore, the coaxial needle can be more accurately placed in those lesions.
In this study, pneumothorax was an independent risk factor for biopsy failure. Pneumothorax increased the difficulty of the coaxial needle reaching the target lesion, which led to difficulties in tissue sampling and to reductions in the number of biopsy specimens. The studies conducted by Gelbman et al. (28) and Takeshita et al. (29) confirmed these findings.
Molecular testing is important to identify driver gene mutations of lung cancer in this era of personalized medicine, as patients with EGFR, ALK, or other activating mutations could be treated with corresponding targeted drugs. Our study demonstrated that gene mutation testing could be successfully performed on 95.45% (168/176) of TCNB specimens. At least 96.8% of samples with two, three, or four passes from a lesion were sufficient for molecular analysis, while only 75.0% of TCNB samples with one pass were sufficient for molecular analysis. Therefore, the recommended number of samples taken from one lesion for molecular analysis was two or more.
Several studies have assessed the adequacy of needle biopsy specimens for molecular testing, but the results varied with the different study strategies and sample sizes. Solomon et al. (30) reported that 89% of needle biopsy specimens acquired using 18- to 20-gauge needles were adequate for EGFR and KRAS sequencing. However, only 18 patients were enrolled in the study. Coley et al. (31) reported that 100% of needle biopsy tissues remained adequate to perform molecular studies. Similarly, only 19 cases underwent needle biopsy in this study. Schneider et al. (32) found that 35 (67%) of 52 needle biopsy specimens contained sufficient tumor cells for molecular testing, including EGFR, KRAS, and ALK testing. However, previous studies have rarely reported the adequacy of needle biopsy specimens from small pulmonary lesions. In our study, we examined a large number of TCNB samples diagnosed as NSCLC and found that the vast majority was sufficient for molecular analysis, although all pulmonary lesions were no more than 3 cm in diameter.
There are certain limitations in this investigation. First, this study was retrospective, and unknown bias existed. In addition, the follow-up observation period of this study was not long enough, which might have had an impact on the final diagnosis. Additionally, there was no control group in this study, and no direct comparison was made against needle biopsy using 20- to 22-gauge cutting needles or CT fluoroscopy-guided lung biopsy; therefore, we cannot evaluate the differences in diagnostic accuracy, complications, and adequacy for molecular testing of those biopsy techniques. Therefore, future prospective studies would be very meaningful.
Conclusions
In sum, for small pulmonary lesions, CT-guided TCNB with an 18-gauge cutting needle has high diagnostic accuracy, and the procedure is safe. A lesion size ≤1 cm, lower lobe lesions, and pneumothorax are independent risk factors for diagnostic failure. Molecular testing for driver gene mutations can be successfully performed on TCNB specimens diagnosed as NSCLC with diameters ≤3 cm.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81372504), the Science and Technology Support Program of Science and Technology Department of Sichuan Province (2016SZ0073), and the International Cooperation Program of Science and Technology Department of Sichuan Province (2014AA022202-2).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the institutional review board of the hospital (Approval number: 2016-85).
References
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-ii54. [Crossref] [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Ng YL, Patsios D, Roberts H, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less. Clin Radiol 2008;63:272-7. [Crossref] [PubMed]
- Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002;225:823-8. [Crossref] [PubMed]
- Zhang HF, Zeng XT, Xing F, et al. The diagnostic accuracy of CT-guided percutaneous core needle biopsy and fine needle aspiration in pulmonary lesions: a meta-analysis. Clin Radiol 2016;71:e1-10. [Crossref] [PubMed]
- Yang W, Sun W, Li Q, et al. Diagnostic Accuracy of CT-Guided Transthoracic Needle Biopsy for Solitary Pulmonary Nodules. PLoS One 2015;10:e0131373. [Crossref] [PubMed]
- Poulou LS, Tsagouli P, Ziakas PD, et al. Computed tomography-guided needle aspiration and biopsy of pulmonary lesions: a single-center experience in 1000 patients. Acta Radiol 2013;54:640-5. [Crossref] [PubMed]
- Yildirim E, Kirbas I, Harman A, et al. CT-guided cutting needle lung biopsy using modified coaxial technique: factors effecting risk of complications. Eur J Radiol 2009;70:57-60. [Crossref] [PubMed]
- Hur J, Lee HJ, Nam JE, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2009;192:629-34. [Crossref] [PubMed]
- Lu CH, Hsiao CH, Chang YC, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 2012;7:143-50. [Crossref] [PubMed]
- De Filippo M, Saba L, Concari G, et al. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol Med 2013;118:1071-81. [Crossref] [PubMed]
- Choi SH, Chae EJ, Kim JE, et al. Percutaneous CT-guided aspiration and core biopsy of pulmonary nodules smaller than 1 cm: analysis of outcomes of 305 procedures from a tertiary referral center. AJR Am J Roentgenol 2013;201:964-70. [Crossref] [PubMed]
- Li Y, Du Y, Yang HF, et al. CT-guided percutaneous core needle biopsy for small (≤20 mm) pulmonary lesions. Clin Radiol 2013;68:e43-8. [Crossref] [PubMed]
- Inoue D, Gobara H, Hiraki T, et al. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: diagnostic yield in 83 lesions. Eur J Radiol 2012;81:354-9. [Crossref] [PubMed]
- Kim GR, Hur J, Lee SM, et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 2011;21:232-9. [Crossref] [PubMed]
- Hiraki T, Mimura H, Gobara H, et al. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest 2009;136:1612-7. [Crossref] [PubMed]
- Laurent F, Latrabe V, Vergier B, et al. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm: results with an automated 20-gauge coaxial cutting needle. Clin Radiol 2000;55:281-7. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer 2016 (v.4.2016). Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed June 2, 2016.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. [Crossref] [PubMed]
- Froelich JJ, Ishaque N, Regn J, et al. Guidance of percutaneous pulmonary biopsies with real-time CT fluoroscopy. Eur J Radiol 2002;42:74-9. [Crossref] [PubMed]
- Nawfel RD, Judy PF, Silverman SG, et al. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology 2000;216:180-4. [Crossref] [PubMed]
- Braak SJ, van Strijen MJ, van Leersum M, et al. Real-Time 3D fluoroscopy guidance during needle interventions: technique, accuracy, and feasibility. AJR Am J Roentgenol 2010;194:W445-51. [Crossref] [PubMed]
- Schulze R, Seebacher G, Enderes B, et al. Complications in CT-Guided, Semi-Automatic Coaxial Core Biopsy of Potentially Malignant Pulmonary Lesions. Rofo 2015;187:697-702. [Crossref] [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Kuban JD, Tam AL, Huang SY, et al. The Effect of Needle Gauge on the Risk of Pneumothorax and Chest Tube Placement After Percutaneous Computed Tomographic (CT)-Guided Lung Biopsy. Cardiovasc Intervent Radiol 2015;38:1595-602. [Crossref] [PubMed]
- Nour-Eldin NE, Alsubhi M, Emam A, et al. Pneumothorax Complicating Coaxial and Non-coaxial CT-Guided Lung Biopsy: Comparative Analysis of Determining Risk Factors and Management of Pneumothorax in a Retrospective Review of 650 Patients. Cardiovasc Intervent Radiol 2016;39:261-70. [Crossref] [PubMed]
- Lee SM, Park CM, Lee KH, et al. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology 2014;271:291-300. [Crossref] [PubMed]
- Gelbman BD, Cham MD, Kim W, et al. Radiographic and clinical characterization of false negative results from CT-guided needle biopsies of lung nodules. J Thorac Oncol 2012;7:815-20. [Crossref] [PubMed]
- Takeshita J, Masago K, Kato R, et al. CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. AJR Am J Roentgenol 2015;204:29-34. [Crossref] [PubMed]
- Solomon SB, Zakowski MF, Pao W, et al. Core needle lung biopsy specimens: adequacy for EGFR and KRAS mutational analysis. AJR Am J Roentgenol 2010;194:266-9. [Crossref] [PubMed]
- Coley SM, Crapanzano JP, Saqi A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: Obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol 2015;123:318-26. [Crossref] [PubMed]
- Schneider F, Smith MA, Lane MC, et al. Adequacy of core needle biopsy specimens and fine-needle aspirates for molecular testing of lung adenocarcinomas. Am J Clin Pathol 2015;143:193-200. [Crossref] [PubMed]