Breast imaging in the young: the role of magnetic resonance imaging in breast cancer screening, diagnosis and follow-up
Introduction
Breast cancer is a disease that knows no boundaries. It can strike women at any age. Doctors may not take young women seriously when they express concerns about breast cancer (1). The wrong perception that young women do not get breast cancer often leads to an initial misdiagnosis. Many breast symptoms and signs in young individuals are dismissed by clinicians and radiologists as cysts or benign breast lesions and they usually adopt a ‘follow up’ protocol.
By the time a lump can be diagnosed in a young woman, it is often large enough and advanced enough to lower the chances of survival. In addition, the cancer may be more aggressive and less responsive to hormone therapy. Breast carcinoma in young patients has been reported to present with more aggressive biologic characteristics and to behave poorly compared with the disease in older patients (2).
Five-year relative survival is lower among women with a more advanced stage at diagnosis. Considering all races, 5-year relative survival is 99% for localized disease, 84% for regional disease, and 23% for distant-stage disease. Larger tumor size at diagnosis is also associated with decreased survival (1). Thus the early detection and diagnosis of breast cancer is thus an emotive issue and a test is required that is both sensitive and specific. In general, regular mammograms are not recommended for women under 40 years of age, in part because breast tissue tends to be denser in young women, making mammograms less effective as a screening tool. In addition, most experts believe the low risk of developing breast cancer at a young age does not justify the radiation exposure or the cost of mammography. Ultrasound (US) although an excellent alternative method for assessing palpable abnormalities in young individuals, yet, it has limitations as a screening modality with a false negative rate ranging from 0.3% to 47% in some series (3).
Breast MRI is no longer an experimental modality, but has attained a solid position in the diagnosis and workup of breast lesions (4). MRI may be particularly helpful in certain situations. This includes high risk patients especially those who have dense breast tissue. Dense breast tissue in young women may obscure signs of malignancy on mammography and limit the evaluation of the true extent of disease (5).
In this review article we will discuss the role of MRI in the screening, diagnosis and follow up of breast cancer in young individuals.
Technique of MRI
MRI utilizes magnetic fields to produce detailed cross-sectional images of tissue structures, providing very good soft tissue contrast. MRI creates images of the breast by measuring changes in the movement of protons in fat and water with the application of changing magnetic fields and by utilizing the differences in tissue relaxation characteristics. Contrast between tissues in the breast (fat, glandular tissue, lesions, etc.) depends on the mobility and magnetic environment of the hydrogen atoms in water and fat that contribute to the measured signal that determines the brightness of tissues in the image. In the breast, this results in images showing predominantly parenchyma and fat, and lesions, if they are present. The use of MRI for breast cancer detection is based on the concept of tumor angiogenesis or neo-vascularity. Tumor associated blood vessels have increased permeability, which leads to prompt take up and release of gadolinium within the first one to two minutes after administration, leading to a pattern of rapid enhancement and washout on MRI. This dynamic rapid enhancement pattern helps to distinguish breast cancers from benign lesions. Thus, contrast enhanced MRI has been shown to have a high sensitivity for detecting breast cancer in high-risk asymptomatic and symptomatic women, although reports of specificity have been more variable (6). This high signal from enhancing lesions can be difficult to separate from fat, leading to the use of subtraction images or fat suppression, or both, to assess disease. Because parenchymal tissue also enhances, but generally more slowly than malignant lesions, and also because contrast can wash out rapidly from some tumors, it is important to look at images at an early time point after contrast injection (typically 1 to 3 minutes). MRI examinations may involve examining images at one time point or, more often, will collect a pre-injection image with sequential sets of images after contrast injection [dynamic contrast-enhanced (DCE)-MRI]. Both the appearance of lesions and, where available, the uptake and washout pattern can be used to identify malignant disease and discriminate it from benign conditions. These techniques, which have been widely employed for assessing symptomatic disease, have recently been shown to provide good sensitivity as a screening tool for breast cancer in women at increased risk based on family history (7-9).
MRI in screening for breast cancer in young females
Breast cancer is diagnosed in over one million women worldwide every year. Until breast cancer can be prevented, early detection offers the best chance for cure (10).
In generic terms, for a screening procedure to be considered useful, it should not only find lesions at an earlier stage, but also it should demonstrate that earlier diagnosis results in some clinical benefit, preferably a reduction in breast cancer mortality (11). Although mammography screening is frequently offered to women with a genetic predisposition to breast cancer at a younger age, the efficacy of this approach is unproven. Preliminary results in such women showed that mammographic screening has a low sensitivity for detecting tumors, especially in carriers of BRCA mutation. These women have a cumulative lifetime risk of developing breast cancer of 21-65%. Women genetically predisposed to breast cancer often develop the disease at young age when dense breast tissue reduces the sensitivity of mammography. Other possible reasons include changes seen on mammography in carriers of BRCA mutation as compared with non carriers of the same age (12-14) (Figure 1).
Owing to the debate regarding the role of MRI as a screening test, the American Cancer Society has outlined recommendations for the use of breast MRI for breast cancer screening. It should be stressed that if MRI is used, it should be in addition to, not instead of, a screening mammogram. This is because although an MRI is a more sensitive test (it’s more likely to detect cancer than a mammogram), it may still miss some cancers that a mammogram would detect. For most women at high risk the ACS recommended screening with MRI and mammograms should begin at age 30 years and continue for as long as a woman is in good health. But because the evidence is limited about the best age at which to start screening, this decision should be based on shared decision-making between patients and their health care providers, taking into account personal circumstances and preferences. The American Cancer Society (ACS) recommended breast MRI screening as an adjunct to mammography for: BRCA mutation carriers and their first-degree relatives; women with a lifetime breast cancer risk ≥20% to 25%; women with a history of chest radiation between ages of 10 and 30 years; and women with predisposing genetic syndromes (e.g., Li-Fraumeni, Cowden). The group felt there was insufficient evidence to recommend for or against MRI screening among women with a personal history of invasive breast cancer or duct carcinoma in situ (15).
In 2010, the European Society of Breast Cancer Specialists (EUSOMA) published a paper evaluating the available evidence regarding clinical value of and indications for breast MRI. This paper reported the results of all the cohort studies investigating the diagnostic performance of different imaging modalities in the surveillance of high-risk women. They recommended that women with a family history suggesting an inherited predisposition to breast cancer should have their risk assessed by an appropriately trained professional group (e.g., genetic counseling). If found to be at high risk (20-30% lifetime risk or greater), these women should be given oral and written information regarding their risk and the risks and benefits of mammography and MRI screening or alternative risk-reducing interventions. If these women accept to be screened by MRI, they should be informed about screening intervals and logistics. This should be determined on the basis of regional or national considerations reflecting an area-specific cumulative risk in the general population, resource availability and practical feasibility. They recommended that annual MRI screening should be available starting at age 30 (16).
Based on several observational studies that have yielded consistent results, the combination of annual magnetic resonance imaging (MRI) plus mammography is now the standard of care for screening women with BRCA mutations who decline risk-reducing mastectomy. Because of its high sensitivity, multiple investigators have studied the potential role of MRI in screening women at high risk. In the past few years, results from eight major clinical trials exploring breast MRI as a screening tool have been published. Combined, the studies included 4,271 patients and found 144 breast cancers detected by MRI, for an overall cancer yield of 3%. The sensitivity of MRI ranged from 71% to 100% across the studies. Although its reported specificity was variable, the call-back rates and risk of benign biopsies were within acceptable limits. In general, patients who underwent breast MRI screening had a 10% risk of being called back, and a 5% risk of having a benign biopsy (17).
A study was conducted to summarize the sensitivity, specificity, likelihood ratios, and posttest probability associated with adding MRI to annual mammography screening of women at very high risk for breast cancer in eleven relevant, prospective, nonrandomized studies that ranged from small single-center studies with only one round of patient screening to large multicenter studies with repeated rounds of annual screening were identified. Characteristics of women that varied across study samples included age range, history of breast cancer, and BRCA1 or BRCA2 mutation status. Studies used dynamic contrast-enhanced MRI with axial or coronal plane images (European studies) or sagittal images (North American studies) that were usually interpreted without knowledge of mammography results. The summary negative likelihood ratio and the probability of a BI-RADS-suspicious lesion (given negative test findings and assuming a 2% pretest probability of disease) were 0.70 (95% CI, 0.59 to 0.82) and 1.4% (CI, 1.2% to 1.6%) for mammography alone and 0.14 (CI, 0.05 to 0.42) and 0.3% (CI, 0.1% to 0.8%) for the combination of MRI plus mammography, using a BI-RADS score of 4 or higher as the definition of positive. The authors concluded that screening with both MRI and mammography might rule out cancerous lesions better than mammography alone in women who are known or likely to have an inherited predisposition to breast cancer (18).
Should we perform MRI of the breast to screen all women?
At this time, MRI is used mostly in breast cancer diagnosis and staging, rather than in screening. Given this impressive ability to detect tumors not found on mammograms, MRI might seem to be a logical choice for breast cancer screening. Yet none of the nationally recognized advisory groups is recommending it for women at average risk. There are several important reasons for this:
- MRI screening is time consuming, requires the injection of intravenous contrast, generates more false-positive results, and has not been shown to impact breast cancer mortality (19);
- High-quality breast MRI is still unavailable everywhere;
- Although screening with MRI may improve survival for women with familial risk of breast cancer, but is expensive. It has been found to be cost effective for women with a BRCA1/2 mutation, it remains unclear whether this is the case for women with a family history of breast cancer without a proven genetic predisposition (20). The projected cost-effectiveness of annual combined screening with MR imaging and screen-film mammography is strongly dependent on the cost of an MR imaging examination and on the underlying breast cancer risk in the women being screened (21);
- Moreover, breast MRI can’t be performed to women who have certain devices in place such as pacemakers or implantable cardioverter-defibrillators;
- The ability of MRI to detect tiny calcifications of early pre-invasive breast cancer (duct carcinoma in situ, or DCIS) is limited;
- Because MRI is so good at picking up any abnormal tissue, whether cancerous or not, it leads to too many negative biopsies;
- False negatives after MRI screening can be attributed to inherent technological limitations of MRI, patient characteristics, quality assurance failures and human error (19);
- False positives can be attributed to the same factors, as well as heightened medical concerns over the consequences of missed cancers. A screening exam is considered to be false positive when its results recommend further testing or a biopsy of a suspicious finding, but no cancer is found. While MRI is more sensitive than mammograms, it also has a higher false-positive rate (it is more likely to find something that turns out not to be cancer). False-positive results during breast MRI screening may have adverse psychological effects. They would lead to unneeded biopsies and other tests in many of the women screened, which can lead to a lot of worry and anxiety (19,22).
MRI in the diagnosis of breast cancer in young individuals
Diagnosis means characterization of detected lesions whether benign or malignant. Staging should pursue this step when malignant pathology is identified.
Sensitivity and specificity contribute to the accuracy of any diagnostic tool. In the case of contrast-enhanced breast MRI, there is strong evidence that the sensitivity is greater than the sensitivity of other techniques of imaging the breast. Currently, breast MR demonstrates a high sensitivity in the range of 93-100%. As many benign lesions also show enhancement or other atypical features on MRI, the primary weakness of contrast enhanced MRI remains in its low specificity, reported to be in the range of 37-97%. However, for the further implementation of diagnostic breast MRI in clinical practice, a reduced overall number of false-positive findings remain a major aim. For sufficient reliable and standardized differential diagnosis of malignant and benign lesions, the characterization of specific features of the lesions is vital (23).
Typically, the first step in evaluating lesion morphology on breast MRI is to classify the lesion as a mass, a focal lesion, or a non-mass-like enhancement. The BI-RADS breast MRI lexicon gives the following clear definitions for mass and non-mass-like enhancement: “Mass—A mass is a three-dimensional space-occupying lesion that comprises one process, usually round, oval, lobular, or irregular in shape”; “Non-mass-like enhancement—Enhancement of an area that is not a mass” (24).
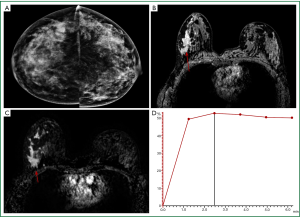
In the case of mass-type lesions there are several parameters that can be used for constructing the differential diagnosis. For example, dark T2 signal, spiculation (morphology), rim or heterogeneous enhancement (texture) and the wash-out kinetic pattern are typical features of malignant lesions; whereas smooth margin (morphology), low and homogeneous enhancement (texture) and a persistent kinetic pattern typically indicate a benign mass (25) (Figure 2).
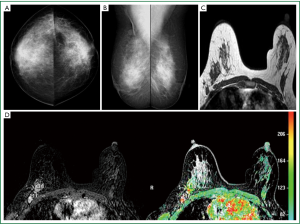
On the other hand, diagnosis of non-mass-like enhancement lesions is much more challenging. Malignant lesions such as duct carcinoma in situ (DCIS) and invasive lobular cancer (ILC) are likely to present as non-mass-like enhancement (Figure 3). Benign fibrocystic changes, which also appear as non-mass-like enhancement, are a frequent finding on DCE-MRI. Unlike mass lesions, non-mass-like enhancement lesions exhibit poorly defined boundaries, leading to difficulty in the analysis of morphology. Furthermore, the malignant non-mass lesions often do not show the typical wash-out pattern in enhancement kinetics, so this very useful diagnosis criterion for mass lesions has a limited diagnostic value for non-mass lesions. Diagnosis of these lesions is challenging because the enhancement of normal tissues and some benign processes, such as fibrocystic change, might show similar appearances (25).
When breast carcinoma is diagnosed, the extent of disease may not be apparent either by palpation or by mammography. Because of its very high sensitivity, MRI is particularly well suited for staging women diagnosed with breast cancer (26).
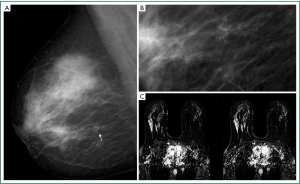
Traditionally, breast cancer was treated with mastectomy, although equivalent long-term survival is obtained with breast-conserving surgery and radiotherapy. Whether a patient is suitable for breast-conserving surgery depends on the size of the mass, particularly in relation to the size of the breast, the presence of multifocal or multi-centric disease, and involvement of the nipple (Figure 4). Multifocal or multi-centric disease has been demonstrated in 31% of patients with known breast cancer. MRI is quite sensitive to multi-focality, provided the scan has been performed to cover the entire breast, or both breasts. Residual breast cancer at the lumpectomy site can result in recurrence. Therefore, successful treatment depends on accurate pre-surgical knowledge of the extent of the disease. Tumor size is under estimated by mammography and ultrasound. Contra lateral occult synchronous tumors could be also identified (27).
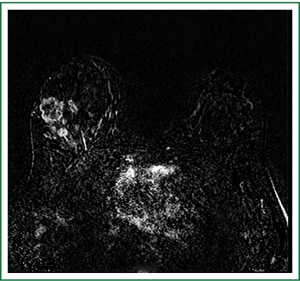
Tumors located posterior in the breast are difficult to evaluate fully with mammography and muscle invasion is often difficult to detect by ultrasound due to acoustic shadowing from the tumor. Breast MRI can also identify chest wall invasion which changes the disease stage to IIIB regardless of primary tumor size. Tumor invasion is identified as muscle enhancement, which may have an infiltrative or mass-like appearance. Muscle enhancement is the only finding which has been shown to reliably indicate tumor invasion. Loss of fat planes between the tumor mass and muscle, and vascular structures extending from the tumor through the muscle, do not indicate tumor invasion (28,29).
Should we perform MRI of the breast to diagnose all cases?
Prudence should be used with the application of Breast MRI in evaluating breast cancer patients due to:
- There is significant overlap of contrast enhancement in benign and malignant breast lesions on MRI;
- The large number of false positive results in additional biopsy in about 4-21% of patients;
- There is significant overlap between normal tissues and malignant tissues;
- Suspicious uptake has been recorded with a variety of benign and benign precancerous conditions;
- Many enhancing features on MRI, particularly those with diffuse or regional distribution that show moderate, progressive-to-stabilized enhancement, do not turn out to be cancer. This pattern can also be associated however, with DCIS, lobular carcinoma, or low grade invasive duct carcinoma. Such findings present a diagnostic dilemma and MR-guided biopsy capabilities are not yet readily available;
- While MRI can demonstrate enhancing lesions on the order of 1-2 mm in size, it is virtually impossible to obtain histo pathologic validation of these small imaging occurrences, making it difficult to determine the true sensitivity of breast MRI (26,27).
MRI in the follow up of breast cancer cases in young individuals
Follow-up post-neoadjuvant chemotherapy
It is becoming increasingly common to treat women with locally advanced disease with neo-adjuvant therapy. Clinical response alone is not a very accurate measure of response to therapy however, and many investigators have pursued imaging to track response. Traditionally, palpation, mammography, and sonography have been used, but edema and necrosis at the tumor site may hinder measurement of the tumor’s true size. Clinical breast exam has been found to underestimate residual disease. MRI is emerging as a very important modality, not only because it can delineate the extent of disease and accurately assess response to therapy, but also because it enables us to look at the morphology of tumors and identify tumor patterns that are distinct at initial presentation (26).
Neo-adjuvant chemotherapy is given to patients after the diagnosis of malignancy has been made but prior to definitive surgical treatment, to decrease the size of the tumor. The change in appearance on post chemotherapy may be decrease in tumor size, change in tumor cellularity, or change in tumor vascularity. The extent of response to chemotherapy and amount of residual tumor determines the treatment options in this setting. Delineating the response poses a clinical challenge. Breast MRI is helpful in demonstrating the true tumor size initially, as well as identifying residual tumor following the completion of neo-adjuvant therapy. Although, MRI is limited by both over- and under estimation of residual disease, it has been shown to correlate more accurately with pathologic specimens pathological complete response (pCR), in the range of 71% to 90%, vs. clinical exam (19% to 60% accuracy), ultrasound (35% to 75% accuracy) and mammography (26% to 70% accuracy). It is important to recognize that even though no residual disease maybe evident by MRI, surgical resection is still required due to the potential under estimation of residual disease. For this reason, it is important to place a tissue marker prior to treatment. MRI provides not only an anatomic evaluation of the tumor but also a physiologic one. As MRI findings are based on the vascularity of the tumor, the effect of chemotherapy agents that inhibit tumor angiogenesis can be seen. Diminished contrast enhancement following chemotherapy would support reduction in tumor vascularity. Decrease in peak contrast uptake and flattening of the contrast uptake curve have been seen in tumors following chemotherapy (30-33) (Figure 5).
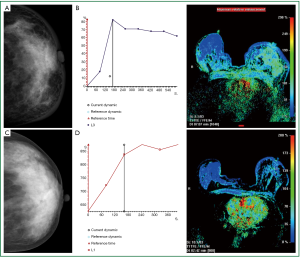
A systematic literature research including forty four studies (2,050 patients) was conducted to examine MRI accuracy in detecting residual tumor, investigates variables potentially affecting MRI performance, and compares MRI with other tests. MRI had higher accuracy than mammography (P=0.02); there was only weak evidence that MRI had higher accuracy than clinical examination (P=0.10). No difference in MRI and ultrasound accuracy was found (P=0.15). The authors concluded that MRI accurately detects residual tumor after neo adjuvant chemotherapy. Accuracy was lower when pCR was more rigorously defined, and specificity was lower when test negativity thresholds were more stringent; these definitions require standardization. They stated that although MRI is more accurate than mammography; however, they recommended further studies comparing MRI and ultrasound (34).
Follow up after breast operations
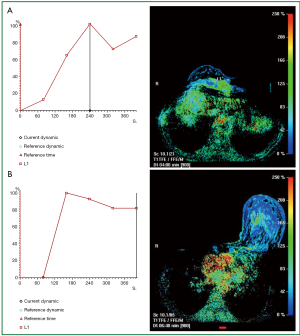
Evaluation of patients who have undergone mastectomy and breast reconstruction is another very difficult issue. As most of the breast tissue has been removed, the site of recurrence is beneath the skin or near the chest wall. These areas are difficult to image with mammography, and post surgical changes can be easily interpreted as malignant. Patients who have been treated with breast conservation therapy with resultant positive surgical margins are typically offered one additional attempt at excision. Mastectomy is usually performed if negative margins are not achieved. Breast MRI in these patients is helpful to identify the extent of residual disease, which may aid in surgical planning for re-excision and may prospectively identify those patients who would ultimately require mastectomy. The purpose of MRI is to detect the presence of multifocal and multi-centric disease as well as to detect bulky residual disease at the lumpectomy site in order to allow directed re-excision. Microscopic residual disease at the surgical margins is known to be present and surgical excision is still required, even if the MRI findings are negative (30).
The evaluation of the tumor bed with MRI is limited, as granulation tissue may enhance in the early postoperative period. Frei et al. determined that the least number of false-positive results were found when MRI was performed between 35 to 42 days following surgery (35). In general, sensitivities ranging from 61% to 86% for detecting residual disease have been reported (36). Studies have shown that the absence of enhancement virtually excludes a recurrence and the presence of enhancement is very specific for tumor even in the radiated breast (30) (Figure 6).
Follow up after breast reconstructive surgery
Breast surgery to rebuild the normal contour of the affected and the contra-lateral unaffected breast to produce a more normal appearance, is considered reconstructive surgery. It is performed following a mastectomy, lumpectomy, or other breast surgery to treat breast cancer. The number of procedures and timing of these procedures varies, depending on the individualized treatment plan devised by the treating physician(s) and the individual and may be impacted by the overall treatment plan for the breast cancer itself (37,38). There are two ways to recreate the breast after a mastectomy: using saline breast implants or the patient’s own tissue (muscle flap reconstruction).
Although imaging with ultrasound and mammography have both been used successfully to evaluate the integrity of implants and detect possible problems over time, MRI is the preferred modality to detect implant rupture (39). Advantages of using MRI to detect implant rupture include imaging with a high sensitivity and specificity, the ability to image the entire implant, and the avoidance of ionizing radiation exposure (40,41).
Conclusions
Contrast-enhanced breast MR imaging is a powerful tool in the breast imaging diagnostic workup especially in high risk young individuals. New evidence on Breast MRI screening has become available since the American Cancer Society last issued guidelines for the early detection of breast cancer in 2003. If MRI is used, it should be in addition to, not instead of, a screening mammogram. The role of Magnetic Resonance Imaging in breast diagnosis will continue to evolve as technology improves and clinical experience with new techniques expands.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Alteri R, Bandi P, Brinton L, et al. Breast Cancer Facts and Figures 2011-2012 a publication of the American Cancer Society, Atlanta, Georgia.
- Gonzalez-Angulo AM, Broglio K, Kau SW, et al. Women age PubMed]
- Rankin SC. MRI of the breast. Br J Radiol 2000;73:806-18. [PubMed]
- Mann RM, Kuhl CK, Kinkel K, et al. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 2008;18:1307-18. [PubMed]
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol 2009;192:390-9. [PubMed]
- Liu PF, Debatin JF, Caduff RF, et al. Improved diagnostic accuracy in dynamic contrast enhanced MRI of the breast by combined quantitative and qualitative analysis. Br J Radiol 1998;71:501-9. [PubMed]
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23:8469-76. [PubMed]
- Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005;365:1769-78. [PubMed]
- Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer 2005;103:1898-905. [PubMed]
- World Health Organization. Cancer Statistics worldwide. Cancer Fact sheet No. 297. Globocan, IARC, 2008.
- Wollins DS, Somerfield MR. Q and a: magnetic resonance imaging in the detection and evaluation of breast cancer. J Oncol Pract 2008;4:18-23. [PubMed]
- Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351:427-37. [PubMed]
- Lord SJ, Lei W, Craft P, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer 2007;43:1905-17. [PubMed]
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 2008;98:1457-66. [PubMed]
- American Cancer Society recommendations for early breast cancer detection in women without breast symptoms. Available online: http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection. Accessed on 30/08/2012.
- Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 2010;46:1296-316. [PubMed]
- Lehman CD. Role of MRI in screening women at high risk for breast cancer. J Magn Reson Imaging 2006;24:964-70. [PubMed]
- Warner E, Messersmith H, Causer P, et al. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med 2008;148:671-9. [PubMed]
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75-89. [PubMed]
- Saadatmand S, Heijnsdijk EA, Rutgers EJ, et al. Cost-effectiveness of screening with additional MRI for women with familial risk for breast cancer without a genetic predisposition. Cancer Res 2012;72:P3-02-09.
- Lee JM, McMahon PM, Kong CY, et al. Cost-effectiveness of breast MR imaging and screen-film mammography for screening BRCA1 gene mutation carriers. Radiology 2010;254:793-800. [PubMed]
- O’Neill SM, Rubinstein WS, Sener SF, et al. Psychological impact of recall in high-risk breast MRI screening. Breast Cancer Res Treat 2009;115:365-71. [PubMed]
- Baltzer PA, Benndorf M, Dietzel M, et al. False-positive findings at contrast-enhanced breast MRI: a BI-RADS descriptor study. AJR Am J Roentgenol 2010;194:1658-63. [PubMed]
- Agrawal G, Su MY, Nalcioglu O, et al. Significance of breast lesion descriptors in the ACR BI-RADS MRI lexicon. Cancer 2009;115:1363-80. [PubMed]
- Newell D, Nie K, Chen JH, et al. Selection of diagnostic features on breast MRI to differentiate between malignant and benign lesions using computer-aided diagnosis: differences in lesions presenting as mass and non-mass-like enhancement. Eur Radiol 2010;20:771-81. [PubMed]
- Esserman L, Wolverton D, Hylton N. Magnetic resonance imaging for primary breast cancer management: current role and new applications. Endocr Relat Cancer 2002;9:141-53. [PubMed]
- Gundry KR. The Application of Breast MRI in Staging and Screening for Breast Cancer. Available online: http://www.cancernetwork.com/breast-cancer/content/article/10165/105413
- Argus A, Mahoney MC. Indications for breast MRI: case-based review. AJR Am J Roentgenol 2011;196:WS1-14. [PubMed]
- Morris EA, Schwartz LH, Drotman MB, et al. Evaluation of pectoralis major muscle in patients with posterior breast tumors on breast MR images: early experience. Radiology 2000;214:67-72. [PubMed]
- Argus A, Mahoney MC. Clinical indications for breast MRI. Applied Radiol 2010;39:32-5.
- Belli P, Costantini M, Malaspina C, et al. MRI accuracy in residual disease evaluation in breast cancer patients treated with neoadjuvant chemotherapy. Clin Radiol 2006;61:946-53. [PubMed]
- Chagpar AB, Middleton LP, Sahin AA, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg 2006;243:257-64. [PubMed]
- Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol 2005;184:868-77. [PubMed]
- Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst 2013;105:321-33. [PubMed]
- Frei KA, Kinkel K, Bonel HM, et al. MR imaging of the breast in patients with positive margins after lumpectomy: influence of the time interval between lumpectomy and MR imaging. AJR Am J Roentgenol 2000;175:1577-84. [PubMed]
- Lee JM, Orel SG, Czerniecki BJ, et al. MRI before reexcision surgery in patients with breast cancer. AJR Am J Roentgenol 2004;182:473-80. [PubMed]
- Diana M, Robb GL, Talavera F, et al. Breast Reconstruction: Nipple-Areola Reconstruction. Updated July 15, 2011. Available online: http://www.emedicine.com/plastic/topic144.htm. Accessed on July 12, 2012.
- Zion SM, Slezak JM, Sellers TA, et al. Reoperations after prophylactic mastectomy with or without implant reconstruction. Cancer 2003;98:2152-60. [PubMed]
- O’Toole M, Caskey CI. Imaging spectrum of breast implant complications: mammography, ultrasound, and magnetic resonance imaging. Semin Ultrasound CT MR 2000;21:351-61. [PubMed]
- Di Benedetto G, Cecchini S, Grassetti L, et al. Comparative study of breast implant rupture using mammography, sonography, and magnetic resonance imaging: correlation with surgical findings. Breast J 2008;14:532-7. [PubMed]
- Gorczyca DP, Gorczyca SM, Gorczyca KL. The diagnosis of silicone breast implant rupture. Plast Reconstr Surg 2007;120:49S-61S. [PubMed]


