Experiences in the management of anastomotic leakages and analysis of the factors affecting leakage healing in patients with esophagogastric junction cancer
Introduction
Esophagogastric junction cancer is one of the most common cancers in human alimentary system worldwide. Surgical resection is so far the most important treatment in the multimodality therapy for resectable esophagogastric junction cancer. However, anastomotic leakage remains a frequent and life-threatening complication with a high mortality after gastroesophageal resection and reconstruction. Up to now, there is no consensus on its classification and treatment regimen yet (1). The objectives of this study was to retrospectively summarize our experiences in the management of anastomotic leakages and analyze the factors that may affect leakage healing in patients with esophagogastric junction cancer after surgical resection in recent 6 years in order to provide useful experiences for the management and prevention of it in the future.
Methods
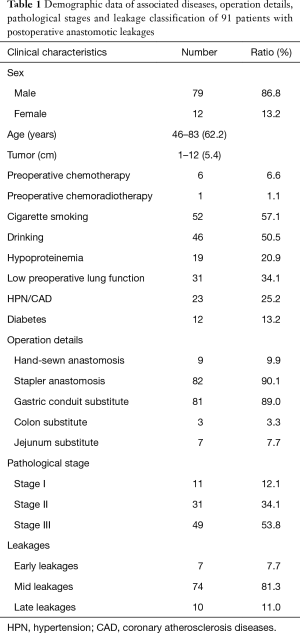
All patients who were diagnosed anastomotic leak and those who stayed in hospital longer than 30 days and may have an anastomotic leakage after surgical resection for esophagogastric junction cancer at our center in recent 6 years were retrospectively analyzed. Among 1,815 surgically treated patients between January 2009 and December 2014, 152 had a longer hospital stay (>30 days). Of those with a prolonged hospital stay, 39 had a longer preoperative preparation due to associated diseases or non-medical reasons but recovered smoothly postoperatively, the other 111 cases had postoperative complications leading to longer hospital stay including anastomotic leakage in 91 cases, other complications but no confirmed leakages in the remain 20 cases, consisting of 1 chylothorax, 1 upper gastrointestinal tract bleeding, 2 post-operative hemothorax, 2 pulmonary infection, 1 thrombus of lower extremity vein, 1 respiratory failure, 2 encapsulated hydrothorax, 5 incision infection and 5 fever with unknown cause. The demographic data including associated diseases, operation details, pathological stages and leakage classification of the 91 patients who experienced anastomotic leaks were listed in Table 1, and all of the patients were pathologically diagnosed adenocarcinoma. The binary logistic regression in SPSS 16.0 was used to analyze the factors that may affect leakage healing. A P value less than 0.05 denoted the presence of statistical significance. The patients were divided into two groups based on the median leakage healing time (40 days) in this series: fast healing group (37 cases) and slowly healing group (54 cases). All factors that may affect the leakage healing were put into analysis by binary logistic regression, including age, sex, smoking index, drinking, pulmonary function, diabetes, neoadjuvant chemotherapy, associated diseases, preoperative albumin level, leakage size, duration of fever, duration of using antibiotics, thoracic drainage, nutrition support, albumin level after developing leakage.
Full table
Results
Partial esophagectomy and proximal gastrectomy with a gastric conduit anastomosed with esophageal stumps was performed through left thoracotomy in 81 cases, and total gastrectomy with jejunum or colon substitute for reconstruction through thoracoabdominal approach was done in 10 cases. All patients’ anastomoses were made in the lower mediastinum under aortic arch by hand-sewn in 9 cases and by circular stapler in 82 cases with an average operating time of 211.5 min, ranging from 60 to 570 min. Twenty-seven (29.7%) had blood transfusion with an average amount of 866.5 mL. Postoperative pathological stage were Ia 5 cases, Ib 6 cases, IIa 13 cases, IIb18 cases, IIIa 13 cases, IIIb 18 cases, and IIIc 18 cases.
Of the 91 leakages, 23 were confirmed by nasal gastroscopy, 18 by iohexol radiography or chest CT showing iohexol leaking into the thoracic cavity, 11 by purulent contents or digestive juice in chest tube, 12 by oral intake of methylene blue, 21 by secondary surgical exploration and methylene blue injection through gastric tube, 6 by CT or ultrasonography guided thoracentesis. Among all leakage patients, the precise location of the leakages was found in 62 cases, consisting of 41 at the anastomosis and 21 at the stumps of the gastric conduit. The leakage size ranges from 0.25 to 3 cm under nasal gastroscopy, with a mean size of 1 cm.
The patients had a fever between postoperative day 1 and day 30, with a median leakage-related fever time at the 8th postoperative day. Eighteen cases developed secondary respiratory failure caused by leakage and were sent to ICU and supported by mechanic ventilation for an average of 7.7 days (range, 1 to 20 days).
All patients except 3 were conservatively treated and drained either by the prophylactically placed drainage tube during operation or a repositioned chest tube into the purulent cavities. Anastomoses were resected and redone in 3 cases due to anastomosis dehiscence. An epidural catheter was placed into the lumen of the chest tube for persistent irrigation of the purulent cavities in 5 patients, using normal saline (NS) and metronidazole. Eighty-nine of 91 patients were supported by both parenteral nutrition (PN) and enteral nutrition (EN) and 2 patients only supported by PN due to unavailable EN catheter (the leakages of these two patients healed up on the 45th and 42th postoperative day). In order to promote leakage healing and protect gastric mucosa, intravenous proton pump inhibitors (PPIs) in all patients and antibiotics in those who had severe infection symptoms were administered according to bacterial culture results.
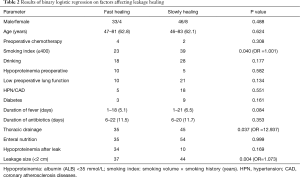
The total leakage rate was 5.0% (91/1,815) in this series, which was 3.9%, 4.6%, 5.3%, 4.2%, 4.8%, and 6.9% from 2009 to 2014 respectively. Three patients died of gastrointestinal hemorrhage with the manifestation of vomiting large amount of blood. The leakage in 65 patients was confirmed to heal up during stay in our center by nasal gastroscopy or iohexol radiography. The time to heal was from 20 to 209 days from the diagnosis of anastomotic leak, with a mean healing time of 40 days. Of the 10 patients who received gastrectomy with jejunum or colon substitute for reconstruction, 9 patients’ leakages were confirmed to heal up from 31 to 129 postoperative days, and 1 patient died of leakage-related complication on the 56th postoperative day after discharge. Twenty-four patients were transferred to other hospitals for continual treatment before healing completely from the 4th to 209th postoperative day. Telephone follow-up was conducted in these 24 patients in the July, 2015. The leakages in 13 patients were confirmed to heal up after discharge and 5 died of leakage-related complications, 6 cases lost follow-up. Therefore, the overall mortality rate in this series was 8.8% (8/91) and the overall healing rate was 85.7% (78/91). The results of multivariant analysis by using binary logistic regression were shown in Table 2.
Full table
The result of multivariant analysis by using binary logistic regression showed that leakage size (OR =1.073, P=0.004), thoracic drainage (OR =12.937, P=0.037) and smoking index ≤400 (OR =1.001, P=0.04) significantly affected the healing time, while drinking history (P=0.177), duration of fever after anastomotic leak developed (P=0.084), and hypoproteinemia after leak (P=0.169) also apparently but not significantly affect the healing time.
Discussion
Esophagogastric junction cancer is one of the most common cancers of the alimentary system in the human being, and surgical resection is still the most important part of the therapeutic regimens for resectable esophagogastric junction cancer. Anastomotic leakage remains the worst complication with high mortality. Up to now, there is no consensus on its classification and treatment yet (2-7). According to the time of appearance, we divided the leakages into three categories: early leakages generally refer to those appearing within 3 post-operative days, and the late leakages usually refer to those presenting after the 14th postoperative day. The leakage occurring between the 4th and 14th postoperative day are usually called mid leakages. Actually, most of the leakages occurred between 8th to 10th days as so-called mid leakages, usually after drinking water or intake of liquid foods. Once anastomotic leakage develops, patients’ internal environment will be disturbed and their nutritional status will get worse and worse, if diagnosed and treated improperly.
Clinical manifestations indicating an anastomotic leakage after surgical resection of esophagogastric junction cancer varies greatly, depending on the size of the leakage and the severity of chest infection (8). Any of the following symptoms and signs such as persistent hyperpyrexia, newly occurred chest pain, chest distress, tachypnea or even a secondary respiratory failure, supraventricular tachycardia, and atrial fibrillation, alert us to the possible presence of an anastomotic leakage and more attention should be paid to the patients. Further examinations should be conducted to confirm or to rule out the leakages. CT with oral radiographic contrast such as Iohexol is a feasible and sensitive way to diagnose anastomotic leakage, and repeated examinations in the suspected cases can increase its sensitivity (9,10). The other optional method is the observing presence of methylene blue in pleural effusion or in pus from the thoracic drainage of the patients with suspected leakages after orally intake of methylene blue (11). According to our experience, nasal gastroscopy is the most effective method to confirm the leakages, it can not only locate the site of the leakage, but also make an assessment of the ischaemia/necrosis of the substitute organs, however it may cause secondary injure such as enlarging the perforation during examination (12). Thoracocentesis according to CT and/or ultrasonography findings is also a useful way to confirm the leakages when combined with oral intake of methylene blue. Once any of the above examinations indicates an anastomotic leakage, a thoracic drainage tube prophylactically placed or newly positioned into the purulent cavities is necessary for the healing of the leaks, and it is always thought to be the most important part in the management of the leakages, as we found that thoracic drainage affected the healing significantly (OR =12.937, P=0.037). If there were no thoracic drainage, the chest infection would not be well controlled and patients’ status could not be improved, eventually, it may not only affect the leakage healing, but may also lead to death. Once the patients’ chest infection is not well controlled, chest CT is indicated and a chest drainage tube should be repositioned. For the patients with separated encapsulated purulent cavities, surgical debridement and a new chest drainage tube with multiple side holes may be necessary. One of our colleague placed another irrigation tube to irrigate the encapsulated purulent cavity at the same time, which was reported effective to promote leakage healing (13), and according to his observation, chest irrigation is very helpful in controlling chest infection and promoting leakage healing.
It was reported in the literatures that individualized management should be applied according to the severity of the symptoms and the leakage size, while the condition of the patients as well as the experience of the surgeons should also be considered (14). Our research discovered that the leakage size affected healing significantly, a smaller leak (<2 cm) is more likely to achieve recovery rapidly. Our experience demonstrated that conservative treatment is effective for most of the patients with asymptomatic or symptomatic small leakages (<2 cm). Conservative treatment in our center usually consists of intravenous use of antibiotics according to germi-culture & susceptibility test until the control of chest infection, PPIs, PN combined with EN, persistent gastrointestinal decompression, chest tube drainage and irrigation. Surgical intervention, which could achieve adequate debridement and chest drainage, is usually indicated for those with symptomatic large leakages or for those conservative management is not effective. It was reported that there was a higher rate of operative mortality in patients underwent surgical intervention as compared with those treated by conservative treatments, although there was no statistic differences in the cure rate between the two groups (15).
Smoking index, refers to smoking volume per day multiplied by smoking year, was thought to be negatively correlated with the healing of anastomotic leaks in our study (OR =1.001, P=0.04). This was not reported before, and we suggest smoking cessation for all patients though it may need to do some further research to exploit the relationship between leakage healing and smoking index. It was reported that anastomotic leakages after surgery for esophagogastric cancer was not significantly relevant to associated diseases such as cardiovascular diseases and diabetes, and our study also showed that associated diseases such as hypertension (HTN), coronary atherosclerosis diseases (CAD) and diabetes mellitus (DM) didn’t affect leakage-healing significantly. Our study showed EN and hypoproteinemia peri-operatively didn’t affect leakage healing significantly, although we all know patients’ nutrition status and supporting is very important for the healing of anastomotic leak, this may attribute to that all the patients were given EN postoperatively and we immediately prescribed intravenous human serum albumin after blood tests indicated hypoproteinemia.
The leakage healing rate was 85.7% and leakage-related mortality was 8.8% in this series, which is much lower than reported 21–35% in literatures (16-18), therefore, the managements for the leakages in our center were proven to be appropriate and effective. Even though leakage is dangerous and fatal, based on our experience, it is still possible to be prevented if some precautious measures are applied. Intraoperatively, a drainage tube with multiple side holes placed along the gastric conduit till the besides of the anastomosis was recommended as a routine procedure at our center, which was very helpful for early observation and drainage of anastomotic leakage in case of it occurs. In addition, reinforcement of the anastomosis with a pedicle omentum is also very helpful to reduce the incidence of anastomotic leakage (19). A continuous prolene reinforcement suture and seromuscular layer embedding for the cutting edges and the stumps of gastric conduit and covering with healthy well-vascularized tissue such as pedicle omentum, plural, pericardium or muscle flap can also effectively reduce the incidence of anastomotic leakages (20,21).
Conclusions
Anastomotic leaks remain to be the most serious complication after operation of esophagogastric junction carcinoma, leakage size, and thoracic drainage are the most important factors affecting the leakage healing. Early recognition and appropriate management of anastomotic leaks can decreased leak-associated mortality and promote leakage healing. A prophylactic drainage tube placement for early identification and control of the leak to reduce the incidence and minimize contamination of the mediastinum is one of the most important ways to promote leakage healing. More attention should be paid perioperatively to the patients who had a smoking index (≥400) and the patients who suffered fever or hypoproteinemia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This was a retrospective study. All patients signed an informed consent before surgery. We reported the study to and gained approval before we started the study from ethical committee of Cancer Hospital, Chinese Academy of Medical Sciences.
References
- Tang H, Xue L, Hong J, et al. A method for early diagnosis and treatment of intrathoracic esophageal anastomotic leakage: prophylactic placement of a drainage tube adjacent to the anastomosis. J Gastrointest Surg 2012;16:722-7. [Crossref] [PubMed]
- Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010;148:831-8; discussion 838-40. [Crossref] [PubMed]
- Veeramootoo D, Parameswaran R, Krishnadas R, et al. Classification and early recognition of gastric conduit failure after minimally invasive esophagectomy. Surg Endosc 2009;23:2110-6. [Crossref] [PubMed]
- Lerut T, Coosemans W, Decker G, et al. Anastomotic complications after esophagectomy. Dig Surg 2002;19:92-8. [Crossref] [PubMed]
- Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157-68. [Crossref] [PubMed]
- Low DE. Diagnosis and management of anastomotic leaks after esophagectomy. J Gastrointest Surg 2011;15:1319-22. [Crossref] [PubMed]
- Rutegård M, Lagergren P, Rouvelas I, et al. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 2012;19:99-103. [Crossref] [PubMed]
- Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;198:536-41; discussion 541-2. [Crossref] [PubMed]
- Strauss C, Mal F, Perniceni T, et al. Computed tomography versus water-soluble contrast swallow in the detection of intrathoracic anastomotic leak complicating esophagogastrectomy (Ivor Lewis): a prospective study in 97 patients. Ann Surg 2010;251:647-51. [Crossref] [PubMed]
- Hogan BA, Winter DC, Broe D, et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc 2008;22:767-71. [Crossref] [PubMed]
- Crestanello JA, Deschamps C, Cassivi SD, et al. Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg 2005;129:254-60. [Crossref] [PubMed]
- Scheepers JJ, van der Peet DL, Veenhof AA, et al. Systematic approach of postoperative gastric conduit complications after esophageal resection. Dis Esophagus 2010;23:117-21. [Crossref] [PubMed]
- Lantos JE, Levine MS, Rubesin SE, et al. Comparison between esophagography and chest computed tomography for evaluation of leaks after esophagectomy and gastric pull-through. J Thorac Imaging 2013;28:121-8. [Crossref] [PubMed]
- Ye HY, Huang WZ, Wu YM, et al. Personalized management of anastomotic leak after surgery for esophageal carcinoma. Chin Med Sci J 2012;27:35-40. [Crossref] [PubMed]
- Guo J, Chu X, Liu Y, et al. Choice of therapeutic strategies in intrathoracic anastomotic leak following esophagectomy. World J Surg Oncol 2014;12:402. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Junemann-Ramirez M, Awan MY, Khan ZM, et al. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg 2005;27:3-7. [Crossref] [PubMed]
- Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005;201:253-62. [Crossref] [PubMed]
- Goldsmith HS, Kiely AA, Randall HT. Protection of intrathoracic esophageal anastomoses by omentum. Surgery 1968;63:464-6. [PubMed]
- Mathisen DJ, Grillo HC, Wilkins EJ, et al. Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg 1988;45:137-43. [Crossref] [PubMed]
- Fekete F, Breil P, Ronsse H, et al. EEA stapler and omental graft in esophagogastrectomy: experience with 30 intrathoracic anastomoses for cancer. Ann Surg 1981;193:825-30. [Crossref] [PubMed]


