Curative resection for lung cancer in octogenarians is justified
Introduction
With an increasing life expectancy in western countries, the mean age of the population is increasing as well as the subgroup of individuals aged >80 years (1,2), who also suffer increasingly from lung cancer (3,4). Lung cancer is the leading cancer and second leading cause of cancer mortality in men and women, respectively, among women worldwide (5). Moreover, the diagnosis of lung cancer is made in more than 40% of patients aged ≥75 years (6). In 2005, Dienemann et al. concluded that the tumour itself rather than the age affects the prognosis of the octogenarian patient (7). Due to the large variability of comorbidities, functional performance, nutritional status, and social resources, which are all associated with aging, the patient population of over 80 years represents a very heterogeneous group. Significant individual variations of such factors are poorly represented by chronological age. Accordingly, the European Respiratory Society, the European Society of Thoracic Surgeons and the American College of Chest Physician recommended that the decision for a surgical procedure should not be based solely on the chronological age (2,4,8-10). Despite a large body of existing literature, it is still strongly debated as to which patient and to what extent a resection should be performed in the surgical management of lung cancer in patients aged over 80 years. To contribute to filling this knowledge gap, we here analysed in a tri-centre, retrospective study the mortality, morbidity, and the short- and long-term follow-up in octogenarians after curative lung resection for non-small cell lung cancer (NSCLC).
Methods
Eighty-eight consecutive patients aged between 80 and 90 years underwent curative lung resection for NSCLC between 2000 and 2013. The mean age at the time of surgery was 82±2.0 years (range, 80–88 years), with 64 male (73%) and 24 female patients (27%). Perioperative risk was classified based on the choice of surgical procedure, quality of life, comorbidities, pulmonary function and the American Society of Anesthesiologists (ASA) score. Whenever possible, preoperative histological diagnosis was obtained by transbronchial needle biopsy, bronchoalveolar lavage, and bronchoscopic or computer-tomography-guided biopsy. Mediastinoscopy and endobronchial ultrasound were not applied routinely. All data were prospectively acquired and retrospectively analysed in three different thoracic departments. To obtain follow-up and survival data, telephone interviews were performed with the patients, their families or the general practitioner. Variables included in the database were age, sex, performance status, ASA score, body mass index, pulmonary function tests [forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and diffusing capacity of the lung to carbon monoxide (DLCO)], preoperative risk factors, type of surgery (wedge resection, segmental resection, lobectomy, bilobectomy, pneumonectomy), chemotherapy treatment, TNM tumour classification, including histology, hospital stay, mortality, morbidity, follow-up and development of tumour-recurrence.
Every patient gave signed informed consent prior to surgery which was approved by the Institutional Ethic Board of University of Zurich.
Statistics
All data were analysed using the Statistical Package for Social Science (IBM SPSS Statistics 22 for Windows). Data were presented as median and range. The survival data were analysed using Kaplan-Meier curves and log-rank test. The statistical frequencies were evaluated using the chi square test and the Fisher’s exact test. A Cox regression analysis was performed to analyse factors determining overall survival. A P≤0.05 was considered as significant.
Results
Patient characteristics and procedures
All 88 patients underwent lung resection with curative intention. The detailed surgical procedures are summarised in Table 1. The preferred procedure was a lobectomy or bilobectomy, while a pneumonectomy was performed only for central tumours and in patients with outstanding performance status (ASA 1 or 2) and respiratory function (both, FEV1 and DLCO ≥70%). Sublobar resection was performed in those patients with impaired performance status (> ASA 3) and severe comorbidities, or in early tumour-stages (cT1, cN0-1). Systematic mediastinal lymphadenectomy was performed on a regular basis during all anatomic resections. The most common comorbidities associated with an increased perioperative risk were the presence of an aortocoronary bypass in eight patients (8.8%), coronary heart disease in five patients (5.6%) and valvular heart disease in four patients (4.4%). The median FEV1, FVC, and DLCO were 2.00 L (range, 0.82–3.66 L), 91% (range, 62–158%), and 69% (range, 12–124%), respectively. In the studied cohort, 5 patients (5.5%) had an ASA score of 1, 50 (55%) an ASA score of 2, 34 (37.3%) an ASA score of 3 and 2 (2.2%) an ASA score of 4. The histological diagnosis was defined according to the criteria of the World Health Organization classification for NSCLC, 7th edition (8,11,12). Adenocarcinoma was diagnosed in 41 patients (46.6%), squamous cell carcinoma in 33 patients (37.5%), large cell carcinoma in 5 patients (5.6%) and other histological types in 9 patients (10.2%). Regarding the final pathological stage, 1 patient (1.1%) presented at stage 0, 53 (60.2%) at stage I, 17 (19.3%) at stage II and 14 (15.9%) at stage IIIA. The definitive histology of the patient at stage 0 revealed adenocarcinoma in situ. In particular, 21 patients (24%) had a positive lymph-node stage, namely 11 patients (12.5%) with pN1 and 10 (11.4%) with pN2 stage. Stage pT3 was found in nine patients (10.2%) and pT4 in two patients (2.3%). No patient received neoadjuvant or adjuvant therapy due to the age with associated risk factors or impaired lung function.
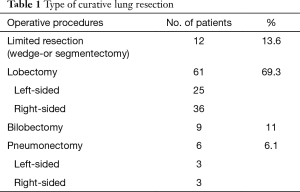
Full table
Mortality and morbidity
The 90-day mortality was 1.1%. One patient with left-sided pneumonectomy died 12 days after surgery due to a postoperative sepsis with right-sided pneumonia, and pulmonary and renal failure. Conversely, 36 patients (40.9%) had an uneventful postoperative course. Of the remaining 51 patients (58%), 41 (46.6%) had only a single complication, 9 (10.2%) had two complications whereas 1 (1.1%) had three complications. Postoperative pulmonary morbidity occurred in 22 patients (25%). In addition, 22 patients (25%) developed cardiovascular morbidity requiring medical—particularly antiarrhythmic—treatment. Twenty-four (27%) patients displayed other complications (cerebrovascular incidents, urinary tract infection). The most frequent complications were atrial arrhythmia in 15 patients (17%), pneumonia in 5 patients (5.6%) and delirium in 5 patients (5.6%) (Table 2).
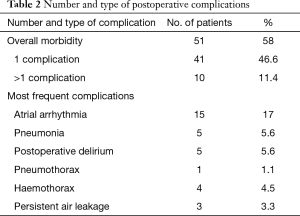
Full table
Out of 88 patients, 9 (10%) were re-operated for haemothorax [4], persistent air leakage [3] bronchial stump insufficiency [1] or pneumothorax [1] within 7 days postoperatively. The patient with the bronchial stump insufficiency underwent an operative debridement and the stump was covered by the omentum. In the three patients with a prolonged air leakage, a surgical closing of the fistula with pulmonary sealant (fibrin glue) or direct suture was performed. The severity of the complications according to the Common Terminology Criteria for Adverse Events is presented in Table 3 (13). The median hospitalisation time was 10 days (range, 5–27 days) and drainage time was 5 days (range, 0–17 days).
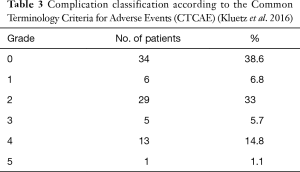
Full table
Follow-up and survival
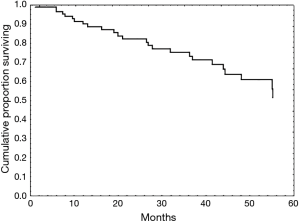
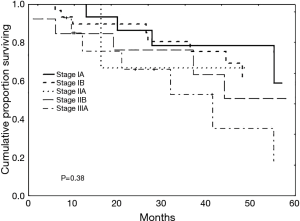
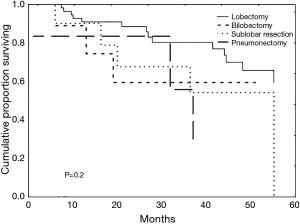
Patients were followed from their time of diagnosis (starting in 2001) to mid-2013. The median follow-up duration was 27 months (range, 1–110 months). During this period, of the 33 patients (37.5%) who died, this was from local recurrences in 4 patients (4.5%), from metastases in 4 patients (4.5%), from non-cancer specific causes in 11 patients (12.5%) or from another malignancy in 1 patient (1.1%). In 13 patients (14.7%), the cause of mortality was undetermined. The median survival time was 40 months (range, 1–110 months) with overall survival rates at 1, 3 and 5 years of 89%, 73% and 45%, respectively (Figure 1). Survival curves according to tumour stage and operative procedure are presented in Figures 2 and 3. Covariates influencing survival are summarised in Table 4. The only independent risk factor was the N-stage (P=0.045). When using Cox multivariate analysis, preoperative FEV1 values and the overall survival were correlated. On the other hand, the significance was lost when the cohort was subdivided into subgroups based on a preoperative 80% FEV1 cut-off and chronic obstructive pulmonary disease (COPD) at stage II, respectively.

Full table
Discussion
In recent years, a series of studies have demonstrated that surgical treatment in selected patients older than 80 years can be successfully performed (2,4,7,14-21). The strength of the present study is, however, that we are able to show that such patients display very low postoperative mortality as well as relatively good early and long-term outcomes even in advanced tumour stages and an equal outcome even after extended resections, including pneumonectomy.
From a prognostic point of view, pulmonary resection remains the best treatment modality for elderly patients with early stage NSCLC (13,22) provided that the mortality is kept low. Advances in pre- and postoperative management of patients, anaesthesia and surgical techniques have led, over recent years, to a significant reduction in the postsurgical mortality and morbidity and increased long-term survival rates. Indeed, Takamochi and colleagues (16) demonstrated equivalent morbidity and mortality rates for elderly patients (over 70 years) compared to younger cohorts. In this series, lobectomy was performed in 85% of the younger group of patients and in 80% of the elderly population. Multivariate analysis revealed that smoking, hypertension, renal insufficiency and DLCO were significant risk factors for the postoperative morbidity among the elderly (16).
Since 2000, the reported perioperative mortality rates have ranged between 0 and 12.5%, mostly dependent on the distribution of TNM stages and the type of surgery performed (14). Compared to these data, our perioperative mortality of 1.1% (in patients aged ≥ 80 years) is extremely low. Dell'Amore et al. reported a perioperative mortality of 6.0% in 83 patients older than 75 years (2). Rivera et al. reported a 30-day mortality of 6.5% in 622 patients aged over 80 years entered in the French registry (4). Shiono et al. reported in 2013 that no mortality occurred in 119 patients older than 75 years (17). However, patients included in the final study underwent only a lobectomy, thus lowering the risk of operation-related mortality.
There is a general consensus that the main causes of perioperative mortality are related to postoperative cardiac or pulmonary complications (2,4,14,16-18). In addition, there is broad evidence that pneumonectomy, particularly on the right side, is a major risk factor for perioperative mortality (2,20,21). We could confirm this trend of complications in as many as 44 patients (50%) who developed cardiac or pulmonary adverse reactions. Likewise, Dell'Amore et al. reported a morbidity rate of 48.6%, with cardiac and pulmonary complications, including postoperative atrial fibrillation, atelectasis, air leakages and prolonged ventilation, as the predominant causes (2). Furthermore, Takamochi et al. (16) described a 42% morbidity rate in 409 septuagenarians; again, the older cohort of patients more frequently developed atrial fibrillation and pneumonia during the postoperative period. Atrial fibrillation was also reported by Dominguez-Ventura to be the most frequent postoperative complication in a population of 379 patients (18).
With regard to possible predictors of postoperative pulmonary morbidity in elderly patients, there are still some inconsistencies: Fanucchi et al. (20) reported that a preoperative FEV1 <1.5 L was not associated with a higher incidence of mortality or morbidity. In contrast, the study by Dell'Amore et al. (2) found FEV1 to be a risk factor for the perioperative mortality. Although, in our study, FEV1-values correlated with the overall survival, the significance was lost when the cohort was divided into subgroups according to the 80% cut-off value.
With regard to postoperative long-term survival, our 5-year survival rate matches well those of other studies ranging from 27% to 57.7% (2,15,17,20,21). The wide range of 5-year survival reported is most likely due to the different proportions of early and late stages of NSCLC as well as the extent of surgical resections. Other confounding factors are selection bias, limited number of patients, incomplete follow-up and the different interpretations of ‘elderly’ (2). The 5-year survival reported by Dell'Amore et al. was at 38% in a cohort of 319 patients with a mean age of 78.1 years (2). In this series, 53.3% of patients had a pathological stage I and 14.7% a pathological stage III NSCLC.
In addition, the long-term survival decreased significantly with increasing tumour stage. Furthermore, Shiono et al. (17) reported a 5-year survival of 57.7% in 119 patients with a mean age of 77 years and a proportion of 71.4% stage I and 15.1% stage III NSCLC. Finally, Okami et al. (15) achieved a 5-year survival of 55.7% in a patient population of 367 clinical stage I patients with a mean age of 82.3 years.
Except for patients who had stages IIA and IIIA lung cancer and did not survive 5 years, our data compare well to the general patient population (14). However, this bias in our study might be due to the small numbers of patients in these cohorts (4 in stage IIA and 14 in stage IIIA).
In our study, the type of surgical treatment, histology, pathologic T-stage, gender and perioperative risk factors showed no correlation with overall survival. Whereas Dienemann et al. (7) and Voltolini et al. (21) reported the tumour stage to be the most important prognostic factor for long-term survival in the elderly, we found only the N-stage as a significant prognostic factor. In addition to tumour stage, long-term survival is influenced by other factors, including pneumonectomy, which is a major risk factor for both perioperative mortality and long-term survival (2,18,20,21). In this context, Voltolini et al. discouraged the performance of pneumonectomy in octogenarians given a significant 25% mortality rate observed in their series (21). Likewise, Dell'Amore et al. (2) reported a mortality rate of 23.3%, with no patient reaching a survival of 5-years. Dominguez-Ventura et al. (18) concluded from their data that patients undergoing pneumonectomy had a poorer long-term survival, with a 10.6% 5-year survival. Accordingly, in our series, no patient who had pneumonectomy survived 5 years. In contrast to Voltolini et al. (21), we attempt to avoid a pneumonectomy whenever possible but do not exclude it a priori. The statistically relevant poorer mortality and long-term survival rates of pneumonectomy may be partially explained by covarying the data with advanced tumour stages as described by Dominguez-Ventura et al. (18).
Maintaining the quality of life constitutes another important issue in octogenarians. Ferguson et al. (23) examined the quality of life of septuagenarians after major lung resection and found that this was not different in such patients compared to younger cohorts, despite a higher number of postoperative complications in the elderly patient population. Stereotactic radiotherapy (SBRT) is the mainstay of alternative therapy in patients who are inoperable and in those who refuse surgery, and this therapy could even be discussed as a curative treatment alternative to surgical resection (24). However, a comparison between surgical and radiotherapy treated patients remains difficult because of the lack of pathologic confirmation of the specimen on radiotherapy treatment. Despite SBRT being usually a well-tolerated treatment, it was not comparable to surgery in different studies (25,26). The European Society for medical oncology clinical guidelines consider SBRT as a first-line treatment in non-operable patients (27). Haasbeek et al. demonstrated an improvement in overall survival in the surgical and radiotherapy groups compared to the patients without treatment (25). Consequently, the decision to subject octogenarians to SBRT is to be made within a dedicated multidisciplinary team with balanced expertise. If possible, a pre-treatment pathological proof, preferably obtained by biopsy, is recommended. If this is not possible and if malignancy is highly likely based on the described criteria, immediate SBRT without histopathological confirmation could be a treatment option (27).
In conclusion, our study demonstrates that curative lung resection should be considered in octogenarians as a first-line therapy. This can be performed up to the extent of pneumonectomy irrespective of the tumour stage, thus offering the best curative therapeutic modality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Every patient gave signed informed consent prior to surgery which was approved by the Institutional Ethic Board of University of Zurich.
References
- Pallis AG, Gridelli C, van Meerbeeck JP, et al. EORTC Elderly Task Force and Lung Cancer Group and International Society for Geriatric Oncology (SIOG) experts' opinion for the treatment of non-small-cell lung cancer in an elderly population. Ann Oncol 2010;21:692-706. [Crossref] [PubMed]
- Dell'Amore A, Monteverde M, Martucci N, et al. Early and long-term results of pulmonary resection for non-small-cell lung cancer in patients over 75 years of age: a multi-institutional study. Interact Cardiovasc Thorac Surg 2013;16:250-6. [Crossref] [PubMed]
- Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am 2000;14:17-23. [Crossref] [PubMed]
- Rivera C, Dahan M, Bernard A, et al. Surgical treatment of lung cancer in the octogenarians: results of a nationwide audit. Eur J Cardiothorac Surg 2011;39:981-6. [Crossref] [PubMed]
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Brown JS, Eraut D, Trask C, et al. Age and the treatment of lung cancer. Thorax 1996;51:564-8. [Crossref] [PubMed]
- Dienemann H, Hoffmann H, Herth F. Thoracic surgery in the elderly. Chirurg 2005;76:126-30. [Crossref] [PubMed]
- The World Health Organization histological typing of lung tumours. Second edition. Am J Clin Pathol 1982;77:123-36.
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Brunelli A, Charloux A, Bolliger CT, et al. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg 2009;36:181-4. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Kluetz PG, Chingos DT, Basch EM, et al. Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book 2016;35:67-73. [Crossref] [PubMed]
- Guerra M, Neves P, Miranda J. Surgical treatment of non-small-cell lung cancer in octogenarians. Interact Cardiovasc Thorac Surg 2013;16:673-80. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Takamochi K, Oh S, Matsuoka J, et al. Risk factors for morbidity after pulmonary resection for lung cancer in younger and elderly patients. Interact Cardiovasc Thorac Surg 2011;12:739-43. [Crossref] [PubMed]
- Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg 2013;16:819-23. [Crossref] [PubMed]
- Dominguez-Ventura A, Cassivi SD, Allen MS, et al. Lung cancer in octogenarians: factors affecting long-term survival following resection. Eur J Cardiothorac Surg 2007;32:370-4. [Crossref] [PubMed]
- Rocco G, Weder W. Lung surgery in the elderly today. Lung Cancer 2013;80:115-9. [Crossref] [PubMed]
- Fanucchi O, Ambrogi MC, Dini P, et al. Surgical treatment of non-small cell lung cancer in octogenarians. Interact Cardiovasc Thorac Surg 2011;12:749-53. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Ligabue T, et al. Short- and long-term results of lung resection for cancer in octogenarians. Asian Cardiovasc Thorac Ann 2009;17:147-52. [Crossref] [PubMed]
- Available online: www.cancer.org/asc/groups/cid/documents/webcontent/003115-pdf.pdf
- Ferguson MK, Parma CM, Celauro AD, et al. Quality of life and mood in older patients after major lung resection. Ann Thorac Surg 2009;87:1007-12; discussion 1012-3. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Antonisse ME, et al. Stage I nonsmall cell lung cancer in patients aged > or =75 years: outcomes after stereotactic radiotherapy. Cancer 2010;116:406-14. [Crossref] [PubMed]
- Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol 2012;23:2743-7. [Crossref] [PubMed]
- Gauden SJ, Tripcony L. The curative treatment by radiation therapy alone of Stage I non-small cell lung cancer in a geriatric population. Lung Cancer 2001;32:71-9. [Crossref] [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. [Crossref] [PubMed]


