Uniportal video-assisted thoracic surgery for major lung resections: pitfalls, tips and tricks
Introduction
Since the first uniportal video-assisted thoracic surgery (uniportal-VATS) major pulmonary resection was reported in 2010 (1), the technique has been spreading worldwide (2).
The uniportal approach allows a direct visualization of the target tissue and of the pulmonary hilum from the front side. The parallel instrumentation achieved through this access mimics the maneuvers performed during open surgery (3-6).
There are certainly some advantages to this approach rather than multiport one: a better lung exposure, direct target view, improved ergonomic, less fulcrum effect and the possibility of digital palpation of the lesion through the small incision (6,7).
Particularly, since only one intercostal space is involved without rib spreading and muscle disruption, some authors reported a decrease in postoperative pain which speeds recovery and reduces hospitalization (8,9), allowing the earlier administration of adjuvant therapy when necessary, with optimal aesthetic results.
The objectives of this work are to set the basic steps for performing major lung resections (lobectomy, bilobectomy and pneumonectomy) by uniportal-VATS and to analyze some common pitfalls that thoracic surgeons could find in their common practice of this technique, providing them practical tips and tricks.
General aspects and basic surgical principles
Uniportal-VATS major pulmonary resections follow the oncological principles of open surgery by anatomical dissection of vascular and bronchial structures as well as complete radical lymphadenectomy in all patients with non-small cell lung cancer (NSCLC).
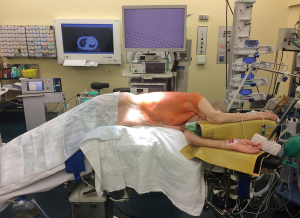
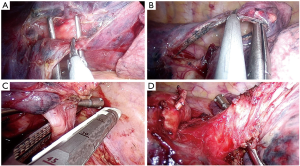
The patient is placed in lateral decubitus position with arms flexed and stretched toward the head on appropriate supports in order to allow room for the surgeon and his assistant to stand in front of the patient. The surgical table is flexed in wedge-shaped position, with patient’s head and legs gently tilted down to allow for better spreading of the intercostals spaces (Figure 1).
The procedure is performed under general anesthesia and double lumen intubation.
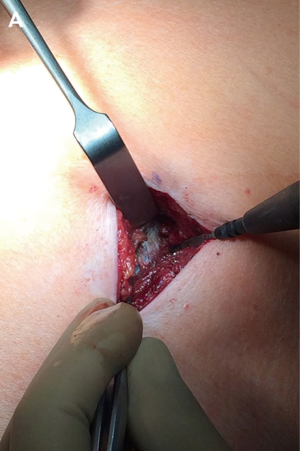
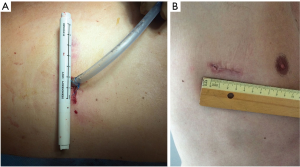
The 2–4 cm single incision is usually made in the 5th or 6th intercostal space but, sometimes, upper and central lesions may require an incision in the 4th space for better exposure and management. The incision is made according to muscle-sparing technique principles: the two bellies of serratus muscle are opened without cutting the fibers and then the intercostal muscle is cut along the upper edge of the lower rib, in order not to destroy its fibers (Figure 2).
Rib spreading is not used but a wound protector is useful for having more space for instruments, for avoiding soiling of the camera and for preventing the risk of wound contamination and infection.
The 10 mm 30° thoracoscope is introduced in the upper part of the incision and both surgeons look at the same screen located opposite to them. The scrub nurse is located on the opposite side (Figure 3A).
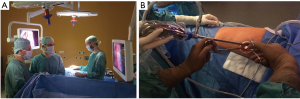
The upper part of the incision represents the fulcrum of the camera shank, and must be as high and as lateralized as possible in order to provide enough space to the first operator to be able to introduce and move instruments. The surgical table should be gently tilted towards the surgeons to allow room for the camera assistance to hold the thoracoscope as high as possible while the tilt of the camera shank does not exceed 30°–40° relative to the horizontal plane (Figure 3B). Such arrangement prevents excessive fatigue from holding the camera and provides a more comfortable position to both surgeons.
Furthermore, when the first surgeon is operating on upper and middle part of chest cavity, the camera assistant must stand in the caudal region of the patient, adjusting the 30°-camera angle to ensure the best vision. When the first operator works on the lower part of the thorax, the assistant stands on the cranial side of patient. Depending on the position with respect to the first operator, the assistant should be cognizant about using the right or left hand to hold the camera, allowing more room for the first operator to work and helping in holding or moving instruments when necessary.
More than two instruments can be introduced simultaneously under the scope in the lower part of the incision. This provides a good angle for reaching and handling any structure within the chest cavity. The instruments are long and curved and are specifically designed for thoracoscopy with proximal and distal articulation to prevent fencing and to allow their simultaneous use.
The stapler must be coming from up and introduced through the lower part of the incision, and must be articulated in the same direction as the thoracoscopic dissector previously used for dissecting.
The small incision enables the identification of the lesion through digital palpation, under camera view, in all necessary cases. No additional purposes for skin incisions such as placement of thoracoscope, graspers, or drains are necessary.
At the end of the operation, the surgical specimens are removed with an Endobag that must be pulled out through the wound with a vigorous but gentle circular movement.
The operation is concluded by a systematic lymphadenectomy of all stations. It can be useful to use an energy device in this phase.
At the end of the procedure, an intrapleural paravertebral intercostal nerve block is performed, infiltrating ropivacaine in 3–4 intercostal spaces above and below the incision, under endoscopic view (Figure 4A,B).
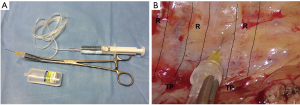
One 24 or 20 Fr chest tube is inserted in the upper part of the incision. The drainage should be placed inside the chest cavity in a curved shape, along the costovertebral sinus, with its tip towards the apex. This position allows for both a good re-expansion of the lung and a spillage of post-operative pleural effusion. In special cases, when two tubes are necessary, one tube can be put in the upper part of the incision and the other one in the lower part.
The incision is closed through suturing the serratus muscle without using any intercostal suture. After closing the subcutaneous tissues, the tube is fixed on the skin suture (Figure 5A,B).
Indications and contraindications
Owing to the increasingly skilled surgeons in uniportal-VATS in recent years, there has been an extension of indications to VATS anatomic pulmonary resections even in case of most common technical difficulties. Therefore incompleteness of interlobar fissures, strong pleural adhesions, preoperative chemo-radiotherapy, obese patients, big size of lesion (10), involvement of lymph nodes, previous thoracic surgery and some cases of centrally located tumors are not supposed to be the contraindications for VATS lobectomy and they do not compromise the short-term results (11-13). Contraindications to uniportal-VATS could be: not sufficient surgeon expertise with this technique, surgeon discomfort and huge tumors that cannot be removed without rib spreading (14).
Operative techniques
After making the incision, as explained above, introducing the camera, inspecting the cavity and confirming the indication to the planned uniportal-VATS major lung resection, proceed as follows.
Right upper lobectomy
The principal steps for a standard right upper lobectomy are:
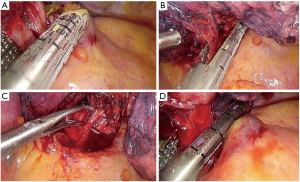
- Identification of the upper vein and anterior arterial trunk (Boyden’s trunk);
- Dissection and division of the anterior trunk, first (Figure 6A);
- Identification of the middle lobe vein and then division of the upper vein (Figure 6B);
- Identification and division of the A2 (Figure 6C);
- Dissection and division of the upper bronchus (after checking inflation of the middle and lower lobe) (Figure 6D);
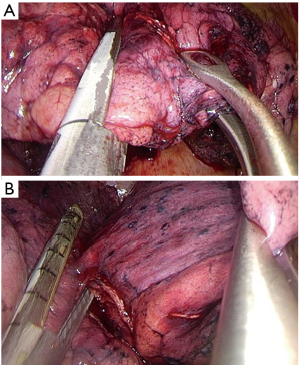
- Dissection of the anterior (Figure 7A) and then the posterior parenchyma (after identification of A6 and A5) (Figure 7B);
- Dissection of the ligament.
Pitfalls, tips and tricks
- In order to expose the artery and the vein, place a clamp on segment 3 parenchyma and retract it posteriorly and inferiorly.
- Be careful with the lymph nodes behind the anterior arterial trunk.
- The direction of the stapler for vein and artery dissection is always towards the lung apex.
- Be careful with anatomical variations of A2 and A3: in approximately 90% of individuals there is an A2 ascending artery that arises from the interlobar artery, sometimes two A2 ascending arteries are described. In 15% of individuals there may not be an A2 ascending artery; in this case the posterior segment is fed entirely from the truncus anterior. A3 arises solely from the truncus anterior in the majority of cases, but additional or replaced supply can also be seen from A2 ascending artery (25% of individuals) or from an A3 ascending artery (in approximately 15%) that arises from the interlobar artery, below the anterior trunk (15,16).
- Small branches of the artery, like A2/A3 could be dissected either by clips (proximal two clips and distal energy device) or vascular stapler.
- Clamp the lung on segment 1 and push it anteriorly and inferiorly in order to expose the posterior side of the bronchus and isolate the lymph nodes. Note that the bronchial artery and visceral pleura are located just above the intermediate bronchus.
- Fissure-less lung: once the correct plane of the artery is identified, insert the jaw of the stapler above the interlobar artery plane and middle lobe vein for cutting the parenchyma between upper and middle lobes anteriorly and between upper and lower lobes posteriorly (Figure 7A,B).
Right lower lobectomy
For performing a standard right lower lobectomy, proceed as follows:
- Dissect the ligament;
- Identify the right lower and middle vein;
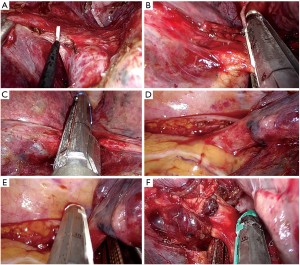
- Dissect the anterior parenchyma between lower and middle lobe (Figure 8A);
- Complete dissection and division of the arterial branches for basal pyramid and A6 (Nelson’s artery) (Figure 8B);
- Dissect the posterior parenchyma (Figure 8C);
- Dissect and divide the lower vein (Figure 8D,E);
- Dissect and divide the lower lobe bronchus (after checking inflation of the middle and upper lobe) (Figure 8F).
Pitfalls, tips and tricks
- After dissecting the ligament, cut the posterior mediastinal pleura up to the upper right bronchus, in order to allow to for better identification of the V6 and bronchial artery.
- To expose the basal pyramid of the artery, catch the parenchyma of segment 7 by clamping and pulling it upwards and posteriorly, in some cases it could be necessary to catch segment 4 too and to push it upwards and posteriorly.
- The direction of the stapler for vein and artery division is always towards the lung basis.
- In case of fissure-less lung or lymphadenopathy in front of the artery, sometimes it’s useful to think about dividing the vein first and then the bronchus and the artery.
- In 80% of individuals, the right lower lobe artery gives off the posteriorly oriented artery to S6 at about the same level as the origin of A4 and A5; however, in 20% of cases, A6 arises from the lower lobe artery as two separate arteries or in rare occasions as 3 separate arteries (15,16).
- If A6 is divided separately from basal trunk, either clips (proximal two clips and distal energy device) or vascular stapler could be used.
- Fissure-less lung: once the correct plane of the artery is identified, insert the jaw of the stapler above the interlobar artery plane (under A4 and A5) for transecting the parenchyma between lower lobe and middle and upper lobes on the anterior and posterior side.
- After the isolation of the right lower bronchus, while holding the lung upwards and anteriorly, place the stapler in a perpendicular way to the bronchus for cutting it (Figure 8F).
Middle lobectomy
The main steps for a standard middle lobectomy are:
- Identification of the right lower, upper and middle vein;
- Dissection of the anterior parenchyma between upper and middle lobe (after identification of the intermediate artery);
- Dissection of the parenchyma between middle and lower lobes (Figure 9A);
- Dissection and division of the middle vein (Figure 9B);
- Identification and division of the A4 and A5 (Figure 9C);
- Identification of the middle lobe bronchus, B6 and the basal pyramid;
- Dissection and division of the middle lobe bronchus (after checking inflation of the upper and lower lobe) (Figure 9D).
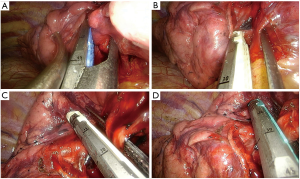
Pitfalls, tips and tricks
- In approximately 50% of individuals A4 and A5 arise as separate branches from interlobar artery, in another 50% they arise as common trunk before dividing. Uncommonly, three separate branches are seen (15,16).
- Be careful with identification of A4–5, keeping in mind the possible anatomical variations of A2, A3, A4 and A5 described previously.
- After dividing the vein, clip the A4 and A5 with the 45° clip applier before cutting (proximal two clips and distal energy device).
- Sometimes for dividing the vein it easier to legate and cut it rather than using vascular stapler.
Upper bilobectomy
Upper bilobectomy is an infrequent operation when compared to other pulmonary lobectomies.
In case the tumor location requires an upper bilobectomy to be radically removed, the already described steps for upper and middle lobectomies can be followed.
Lower bilobectomy
For performing a standard lower bilobectomy, proceed as follows:
- Identify the right lower, middle and upper vein;
- After dissection of the ligament, follow the division of the lower and middle vein (Figure 10);
- After identification of the intermediate artery, dissect the anterior parenchyma between upper and middle lobe (Figure 11A);
- Identify A4 and A5, then A2 and A6;
- Divide the intermediate artery above A4–A5, preserving A2 (Figure 11B);
- Complete the dissection of the posterior parenchyma between upper and lower lobe (Figure 11C);
- Identify the intermediate bronchus and middle lobe bronchus;
- After checking inflation of the upper lobe, divide the intermediate bronchus (Figure 11D).
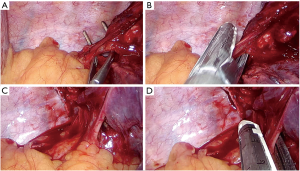
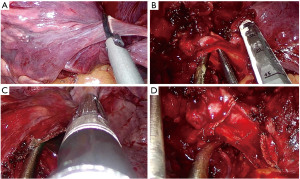
Pitfalls, tips and tricks
- In case of fissure-less lung or bad lymphadenopathy or after radiochemotherapy, sometimes it is useful to first dissect and cut the vein, then bronchus and at finally the artery and the parenchyma.
- Grasp the lung parenchyma and push the lung upwards and posteriorly in order to have enough space and to have a good explosion for cutting the bronchus.
- If you face any difficulty when dividing intermediate artery, you can cut each branch (A4, A5, A6 and piramidal branches) either by clips (proximal two clips and distal energy device) or by vascular stapler.
- After cutting the ligament, it is helpful for the preparation of the intermediate bronchus to open posterior pleura up to the origin of upper bronchus.
Right pneumonectomy
The principal steps for a standard right pneumonectomy are:
- Dissection of the ligament;
- Identification of the upper, middle and lower veins;
- Dissection and division of the main artery (as for left pneumonectomy);
- Division of the veins (as described previously for upper right lobectomy and lower bilobectomy, Figure 6B and Figure 10);
- Dissection and division of the main bronchus (as for left pneumonectomy).
Pitfalls, tips and tricks
- If the common trunk of the artery is too short or intrapericardial so that there isn’t enough space for placement of the stapler it is suggested to identify and dissect the upper trunk first and then dissect the right artery above A2.
- The direction of the stapler for dissection of the artery is always towards the apex.
- It is important to keep in mind that sometimes open bronchoplastic operation is better than minimally invasive pneumonectomy.
- In case you need to protect the bronchial stump by a flap you can use the intercostal muscle of the same intercostal space of the incision, after preparing and cutting it under endoscopic vision.
Left upper lobectomy
The principal steps for a standard left upper lobectomy are:
- Identification of the upper vein and lower vein after dissection of the ligament;
- Identification, dissection and division of the anterior artery (Figure 12A);
- Division of the upper vein (Figure 12B);
- Dissection of the anterior parenchyma (Figure 12C);
- Dissection of the posterior parenchyma (after identification of A6) (Figure 12D);
- Identification and division of the lingular arteries;
- Dissection and division of the remaining arteries (Figure 12E);
- Dissection and division of the upper bronchus (after checking inflation of the lower lobe) (Figure 12F).
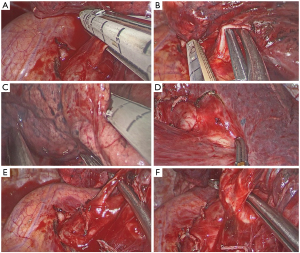
Pitfalls, tips and tricks
- Pay attention to the common left vein [23.9% of cases, (17,18)]!
- Be careful with the lymph nodes behind the anterior artery.
- Divide the vein first for having more space in exposing and dissecting arterial branches in case of lymphadenopathy or strong adhesion as after RT/CT.
- The left upper lobe arterial branches may arise in fairly random order, and they frequently form small trunks of two or small branches. Two to seven branches may originate from the proximal left pulmonary artery to supply upper lobe. A3 subsegmental arteries can arise as a common trunk or separate branches from the left pulmonary artery. In 40% of individuals, A3 or a subsegmental branch of it can have a common trunk with part of all A1 + A2 (15,16).
- The lingular arteries arise from the anterior aspect of left interlobar artery in 90% of cases; they may arise as a single trunk or separate arteries (15,16).
- When dividing the arterial branches, use either proximal two clips and distal energy device or vascular stapler.
- Before the dissection of the upper bronchus, be careful with cutting all arterial branches behind the bronchus (Figure 12E).
- Fissure-less lung: once the correct plane of the artery is identified, insert the jaw of the stapler above the interlobar artery plane for cutting the parenchyma of the fissure on the anterior and posterior side.
Left lower lobectomy
For performing a standard left lower lobectomy, proceed as follows:
- Dissect the ligament;
- Identify the upper vein and lower vein;
- Dissect the anterior parenchyma (Figure 13A);
- Identify and divide the arterial branches to basal pyramid with or without A6;
- Dissect the posterior parenchyma (Figure 13B);
- Divide the lower vein (Figure 13C);
- Dissect and divide the lower bronchus (after checking inflation of the upper lobe) (Figure 13D).
Pitfalls, tips and tricks
- Pay attention to the common left vein [23.9% of cases, (17,18)]!
- In case of fissure-less lung or lymphadenopathy in front of the artery, sometimes it’s useful to think about dissecting the vein, bronchus and then the artery.
- In 70% of individuals, A6 originates usually as a single trunk from the interlobar artery at or above the lingular arteries. Frequently, A6 arises as two or more separate branches; occasionally A6 arises from a lingular artery (15,16).
- A6 could be divided either by clips (proximal two clips and distal energy device) or by a vascular stapler.
- After the isolation of the left lower bronchus, while holding the lung upwards and anteriorly, place the stapler in a perpendicular way to the bronchus for cutting it (Figure 13D).
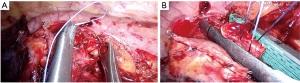
- In case of central or endobronchial tumor and not having enough space for placing the stapler, the bronchus could be cut upstream the tumor—on free borders—by scalpel. Then the bronchial stump could be closed by stapler, after holding and pulling it by two traction stitches, like in Figure 14.
Left pneumonectomy
For performing a standard left pneumonectomy, proceed as follows:
- Dissect the ligament;
- Identify the upper and lower vein;
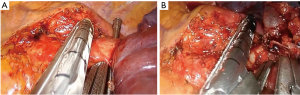
- Identify and divide the main artery (Figure 15A);
- Dissect and divide the upper vein and lower vein;
- Divide the main bronchus (Figure 15B).
Pitfalls, tips and tricks
- It is important to keep in mind that sometimes open bronchoplastic operation is better than minimally invasive pneumonectomy.
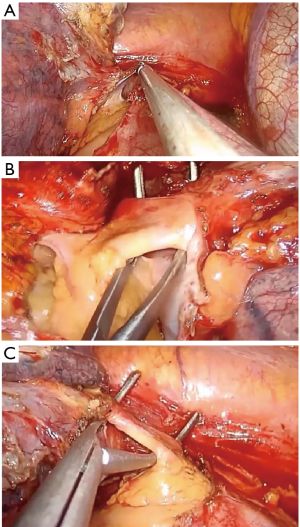
- If you face difficulty in identifying the common trunk of the artery due to bad lymphadenopathy or a very short trunk, open the pericardium (Figure 16A) and prepare it from the inside. The same procedure could be done for dissection of the veins (Figure 16B,C).
- In case you need to protect the bronchial stump by a flap you can use the intercostal muscle of the same intercostal space of the incision, after preparing and cutting it under endoscopic vision.
Comments
According to our experience, uniportal VATS is a feasible and safe technique not only for surgeons specialized in anterior approach but also for the surgeons with most experience in lung surgery by posterolateral access (19).
The use of special and suitable instruments in performing this technique is strongly suggested to optimize surgical maneuvers and reduce operating times. In fact these instruments, specifically designed for uniportal-VATS, with proximal and distal articulation and slight curvature, reduce fencing and allow the simultaneous use of multiple instruments through one small incision. Despite suggestions given in this paper, sometimes the first steps with this approach for major lung resections can be difficult without having a uniportal-VATS experienced surgeon present during operation. However attending wetlabs and hands on courses available worldwide as well as visiting experienced uniportal-VATS centers can be a good opportunity for learning or improving this technique which possesses a rapid learning curve.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D. Keep calm and think uniportal. Video-assist Thorac Surg 2016;1:15. [Crossref]
- Rocco G. One-port (uniportal) video-assisted thoracic surgical resections--a clear advance. J Thorac Cardiovasc Surg 2012;144:S27-31. [Crossref] [PubMed]
- Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach. ScientificWorldJournal 2012;2012:780842. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Thoracoscopic lobectomy through a single incision. Multimed Man Cardiothorac Surg 2012;2012:mms007. [Crossref] [PubMed]
- Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5 Suppl 3:S214-6. [PubMed]
- Shi Z, Chen C, Jiang S, et al. Uniportal video-assisted thoracic surgery resection of small ground-glass opacities (GGOs) localized with CT-guided placement of microcoils and palpation. J Thorac Dis 2016;8:1837-40. [Crossref] [PubMed]
- Akter F, Routledge T, Toufektzian L, et al. In minor and major thoracic procedures is uniport superior to multiport video-assisted thoracoscopic surgery? Interact Cardiovasc Thorac Surg 2015;20:550-5. [Crossref] [PubMed]
- Hao Z, Cai Y, Fu S, et al. Comparison Study of Post-operative Pain and Short-term Quality of Life between Uniportal and Three Portal Video-assisted Thoracic Surgery for Radical Lung Cancer Resection. Zhongguo Fei Ai Za Zhi 2016;19:122-8. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Pischik VG. Technical difficulties and extending the indications for VATS lobectomy. J Thorac Dis 2014;6:S623-30. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Gonzalez-Rivas D, Yang Y, Sekhniaidze D, et al. Uniportal video-assisted thoracoscopic bronchoplastic and carinal sleeve procedures. J Thorac Dis 2016;8:S210-22. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic lobectomy. J Thorac Dis 2013;5 Suppl 3:S234-45. [PubMed]
- Pearson FG, Cooper JD, Deslauriers J, et al. Anatomy of the lung. Thoracic Surgery, 2nd edition. New York: Churchill Livingstone, 2002:427-41.
- Shields TW. Surgical anatomy of the lungs. In: Shields TW, LoCicero J, Ponn RB, et al. Editors. General thoracic surgery, 6th edition. Philadelphia: Lippincott Williams&Wilkins, 2005:59-73.
- Healey JE Jr. An anatomic survey of anomalous pulmonary veins: their clinical significance. J Thorac Surg 1952;23:433-44. [PubMed]
- Irene A, Theodoros K, Konstantinos N. Pulmonary vein anatomical variation during videothoracoscopy-assisted surgical lobectomy. Surg Radiol Anat 2017;39:229-31. [Crossref] [PubMed]
- Ismail M, Helmig M, Swierzy M, et al. Uniportal VATS: the first German experience. J Thorac Dis 2014;6:S650-5. [PubMed]