Sentinel lymph node mapping in patients with operable non-small cell lung cancer
Introduction
The presence of metastatic disease in the mediastinal lymph nodes has been shown to be the strong predictor of survival after resection of non-small cell lung cancer (NSCLC) (1-3). Approximately 40% of patients with early-stage lung cancer have a recurrence of the tumour and the 5-year survival after complete resection of stage I tumors is only 60% to 70% (1). Three probable reasons may suggest the high rate of metastasis for early stage tumors first, an inadequacy of the lymphadenectomy; second, a pathologic failure in that the appropriate lymph nodes were removed to microscopic disease; third, there may be hematologic or pleural dissemination (4). However, micrometastatic tumor cells are not detected by current clinical staging examinations and conventional histopathological methods and have already spread to loco-regional lymph node metastasis at the time of surgery. Our knowledge is that adequate surgical dissection of the regional lymphatics improves treatment results, and the morbidity of a complete mediastinal node dissection for lung cancer is not excessive. However specially extent lymph node dissection is still controversy (5-9).
The sentinel lymph node (SLN) is the first node draining a tumor. Assessment of the SLN to predict metastases in the regional nodal basin has proven valuable in staging with melanoma and breast cancer. Unfortunately due to a specific anatomical considerations in the lung SLN with radiocolloid in lung cancer was abandoned. The main cause of such an attempt was technical problems with this method caused by very intense vascular supply in the lung, inability to inject the tracer with a big particle before the operation due to logistic circumstances etc. The objective of this prospective study is therefore to evaluate the feasibility of SLN mapping in operable NSCLC using Technetium 99m nanocolloid (Tc 99m) and a hand-held gamma detection probe and we used a lead sheet between tumor and gamma probe to prevent the “shine through” effect of the background (remaining lung tissue) as new concept.
Materials and methods
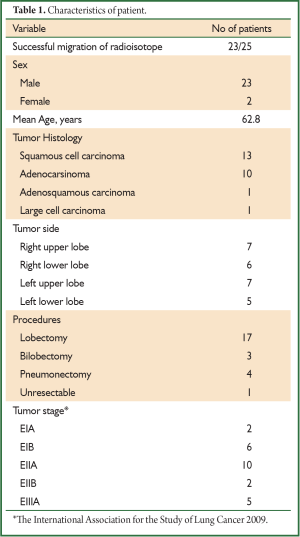
The study for SLN identification was approved by the ethical committee of Trakya University, Faculty of Medicine in October 2008. Informed consent was obtained from all patients after their discussion with the operating surgeons of the risks and benefits. Eligible patients had operable NSCLC and were candidates for resection with mediastinal lymph node dissection. Routine preoperative assessment of operability included CT scan of chest, negative positron emission tomography (PET) for lymph node and systemic metastasis. Twenty-five patients [23 men, two women; mean age, 62.84 years (47-81 years)] were enrolled consecutively into this study between 2009 and 2011. The standard preparations were made for thoracotomy and resection.
Intraoperative SLN mapping technique
At thoracotomy, if necessary, a frozen section of the tumor undertaken to ensure diagnosis, and then a total dose of 1 mL of Tc-99m nanocolloid (particle size: over 95%, 80 nm) including 0.25 mCi in four divided proportion was injected at into each quadrant of lung tissue surrounding the tumor intraoperatively.
Intraoperative radioactivity counting at the nodal stations started a mean of 1 h (range, 45-60 min) after the injection with the Tc-99m nanocolloid. While this interval we waited for the radioisotope migration without performing any procedure and covered with a sterile drap in order to avoid heat loss. The migration of the Tc-99m nanocolloid was measured by the lymphatics by a hand-held gamma probe. Background levels were initially recorded in the thorax with hand-held gamma probe counter after calibration. After 45-60 minutes, radioisotope was considered successful if a specific nodal station measured counts per second greater than three time background values. The value of counts per second of the primary tumor and intrathoracic nodal stations were recorded in vivo and ex vivo after dissection. The ex vivo measurements were done to estimate the effect of shine accurate. So, hilar lymph node stations were measured after removal from the resected lung. Unlike other studies, we measured lymph node activity that by putting 10 cm × 10 cm lead sheet between tumor and gamma probe. This technique has allowed a significant decrease in background radiation from the tumor, enhancing our ability to identify a SLN station in vivo. Anatomical resections were performed with mediastinal lymph node dissection according to the Revisions in the International System for Staging Lung Cancer (1). Careful dissection was performed in the proximity of bronchial structures and the lymph nodes.
Results
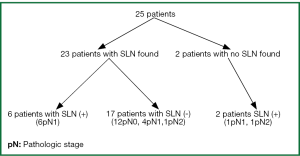
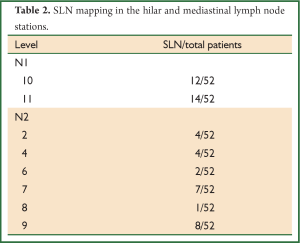
The postoperative pathologic TNM staging revealed T1aN0M0 (8%) in two patients, T1aN2M0 (4%) in one patient, T1bN1M0 (8%) in two patients, T2aN0M0 (24%) in six patients, T2aN1M0 (24%) in six patients, T2aN2M0 (8%) in two patients, T2bN0M0 (8%) in two patients, T2bN1M0 (4%) in one patient, T3N0M0 (4%) in one patient, T3N1M0 (4%) in one patient, T4N0M0 (4%) in one patient, respectively (Table 1). Successful migration was observed in 23 of the 25 patients (92%) and the lymph nodes were successfully identified. A total of 52 SLNs was detected. SLN was identified a single in 10 (76.92%) patients, two lymph node in six patients (9%), three lymph node in one patient, four lymph node in three patients, five lymph node in three patients respectively (Table 2). SLN was not found in two patients (8%) (pN1; n=1, pN2; n=1). Six out of 25 patients (24%) were positive for metastatic involvement after full histopathologic evaluation (pN1; n=6), whereas seventeen patients were negative SLN (pN0; n=12, pN1; n=4, pN2; n=1) (Figure 1). As a result, the false negative rate was 29% in our study (n=5). In two patients, SLN which could not be found, whereas the histopathologic analysis revealed positive nodes located in level 5 and 10 respectively and so, the false positive rate was 8%. However, the sensitivity, specifity, accuracy rate, positive predictive value (PPV), negative predictive value (NPV), 55%, 86%, 72%, 75%, 71%, respectively.
Full Table
Full Table
Discussion
SLN mapping techniques introduced first by Cabanas (10) in 1977 and have been applied to most solid tumors as melanoma and breast cancers. The SLN is the first node draining a tumor and the verification of the SLN concept in melanoma has allowed the identification of patients who may benefit from lymphadenectomy, and prevented unnecessary lymphadenectomy in those with no affected SLN. At the same time, this strategy may shorten the duration of surgery, avoid potential immunologic effects of extensive lymphatic dissection and with the accurate identification of positive nodes gained therapeutic importance and a negative sentinel node reflects a node negative mediastinum. The first reported use of intraoperative radioactive tracer was in 2000 years with Technetium-99 sulfur (11). The relatively low experience with SLN technique in NSCLC reflects limited interest of thoracic surgeons in this field. Nevertheless, the results achieved in pilot studies of NSCLC provide a promising basis for further investigations (4,7,8,12). Different criteria for the definition of SLN and different radiocolloids were analyzed to propose the best methods for further clinical development. Although, in recent reports were not found differences in either sensitivity or NPV among the four most commonly used radiocolloids, those with a particle size less than 100 nm showed slightly better results (12). So, we also decided to use Tc 99m nanocolloid, particle size <80 nm. SLNs were successfully identified in 23 of the 25 patients (92%). In our study, compared to the 82% found by Liptay et al. (11), the 64.3% found by Sugi et al. (13), the 96% found by Melfi et al. (14), the 54.2% founded by Tiffet et al. (4) using only radioisotope SLN mapping. Thus, our findings consistent with other’s series. The “shining through” phenomenon sometimes interfered with use of the gamma probe in the lymphatic region. Therefore, we measured lymph node activity that by putting 10 cm × 10 cm lead sheet between tumor and gamma probe. This technique has allowed a significant decrease in background radiation from the tumor, enhancing our ability to identify a SLN station in vivo. The most significant point of sentinel nodal biopsy is to identify negative sentinel nodes and be cleared that the distal nodal involvement is also negative. In the 23 patients in whom a sentinel node was identified, the sentinel node was negative in 17 and no metastases were found in the mediastinal nodes (12 of 17 patients, 71%). In the six patients in whom the sentinel node was positive, all were found to have metastases in the mediastinum (6 of 6 patients, 100%). Thus, sentinel node assessment appears to be an accurate way to stage the mediastinal nodal evaluation. However, it is now known that the mediastinal lymph node involvement without spread to the intraparenchymal and hilar nodal basins has been termed skip metastases. This phenomenon occurs in between 25% and 40% of resected N2 diseases. However, the skip metastases could not be detected in our study. The false negative rate was 29%. In 4 of 5 patients (80%) with false negative results, lymph node metastasis was found in hilar lymph node (No.10, 12, 13 stations) of which radioactivity was not measured intraoperatively due to technical problems. Mediastinal lymph node involvement was noted in one patient (No.7 station) The false negative SLN rate for Little et al. (15) and Schmidt et al. (16) was 0%, while it was 5% for Liptay et al. (11) and 3.8% for Melfi et al., (14) 14% Tiffet et al. (4). Our findings did not confirm these results and higher than others. The reason for this might also lead to false- negative results due to the hilar lymph node stations’ shine-through from the tumor radio-activity. Our findings confirmed these concerns despite the lead barrier placed between tumor and lymph nodes. Although the ex vivo measurement is more accurate than the in vivo measurement, SLN identification before lymph node dissection by in vivo measurement is necessary for SLN prediction surgery. Other factors contributing to this outcome may be dissection often causes extravasation of the blood with contamination of the area of measurement by the radiocolloid and difficult to perform when the central tumor is close to the lymph node (12).
In recent studies, sensitivity was over 80%, while it was 55% for our studies (11,12,14). We suggest that, our low sensitivity may relate to the small number of patients and the limited use of immunohistochemistry in this study, confined to little level from each block.
In conclusion, intraoperative detection of the SLN with Tc-99m nanocolloid techniques in specially in peripheral NSCLC is easy and safe. One major limitation of the technique is its difficulty to detect SLNs located in stations 12 or 13, 14. It is not yet sufficiently sensitive to have a role in reducing the extent of nodal dissection. Our initial experience with this technique has yielded that further prospective studies are needed to confirm application and validation in larger groups of patients and the detection and shining prevention methods in order to improve the technique.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg 1988;96:440-7. [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [PubMed]
- Tiffet O, Nicholson AG, Khaddage A, et al. Feasibility of the detection of the sentinel lymph node in peripheral non-small cell lung cancer with radio isotopic and blue dye techniques. Chest 2005;127:443-8. [PubMed]
- Graham AN, Chan KJ, Pastorino U, et al. Systematic nodal dissection in the intrathoracic staging of patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 1999;117:246-51. [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [PubMed]
- Wu Yl, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6.
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65; discussion 365-6. [PubMed]
- Bollen EC, Van Duin CJ, Theunissen PH, et al. Mediastinal lymph node dissection in resected lung cancer: morbidity and accuracy of staging. Ann Thorac Surg 1993;55:961-6. [PubMed]
- Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [PubMed]
- Liptay MJ, Masters GA, Winchester DJ, et al. Intraoperative radioisotope sentinel lymph node mapping in non-small cell lung cancer. Ann Thorac Surg 2000;70:384-9; discussion 389-90. [PubMed]
- Rzyman W, Hagen OM, Dziadziuszko R, et al. Intraoperative, radio-guided sentinel lymph node mapping in 110 nonsmall cell lung cancer patients. Ann Thorac Surg 2006;82:237-42. [PubMed]
- Sugi K, Fukuda M, Nakamura H, et al. Comparison of three tracers for detecting sentinel lymph nodes in patients with clinical N0 lung cancer. Lung Cancer 2003;39:37-40. [PubMed]
- Melfi FM, Chella A, Menconi GF, et al. Intraoperative radioguided sentinel lymph node biopsy in non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:214-20. [PubMed]
- Little AG, DeHoyos A, Kirgan DM, et al. Intraoperative lymphatic mapping for non-small cell lung cancer: the sentinel node technique. J Thorac Cardiovasc Surg 1999;117:220-4. [PubMed]
- Schmidt FE, Woltering EA, Webb WR, et al. Sentinel nodal assessment in patients with carcinoma of the lung. Ann Thorac Surg 2002;74:870-4; discussion 874-5. [PubMed]