Sexuality and breast cancer: prime time for young patients
Introduction
Sexuality is a basic and important domain of human experience (1) that can be damaged during and following cancer treatment (2). Several studies refer to sexual functioning as an important domain of health-related quality of life (HRQoL) of oncologic patients (3-6), that, nonetheless, is lacking proper undertaking by healthcare providers (4).
The risk of sexual dysfunction is even of greater importance among young cancer patients and survivors (4,7), with young breast cancer (BC) patients at particularly high risk (4,5,7). The reasons are: (I) the growing number of diagnosis of BC among premenopausal women all over the world (8), who already comprise 25% of all diagnosis of breast cancer (9); (II) a higher fragility of young women regarding their sexual self-conception and body image (versus their older counterparts); (III) the closely related comorbidities of premature menopause and infertility, caused by the disease and its treatments, that frequently develop concomitantly with sexual dysfunction; (IV) the developmental and relational period in these women’s lives, where they are struggling to form stable peer and intimate partner relationships, highly engaged in studies or early professional careers, gaining/reinforcing their personal and financial independence, and having to manage and integrate all this with BC diagnosis and treatment (5,8-12).
The scope of this review will be the pathophysiology of female sexual function, emphasizing the specific risk factors for abnormal functioning in young breast cancer patients, its assessment tools, management (pharmacological and non-pharmacological treatment interventions) and global strategies for improvement of sexual health management in breast cancer oncology practice.
Sexual health and sexual dysfunction in young women with breast cancer
The sexual experience is complex, involving many internal and external factors. There are various definitions of sexuality and sexual functioning, namely the one by the World Health Organization (13), “as a state of physical, mental and social well-being in relation to sexuality… as well as the possibility of having pleasurable and safe sexual experiences, free of coercion, discrimination and violence”.
The most widely-know model of sexual functioning (for both sexes) was developed by Masters and Johnson in 1966 (14,15) and consists of four phases: (I) excitement phase (initial arousal) with feelings of sexual pleasure accompanied by physiologic changes (genital vasocongestion, increase in respiratory rate, heart rate and blood pressure); (II) plateau phase with maximal arousal and muscular tension; (III) orgasm, with the peak of sexual pleasure, accompanied by rhythmic contractions of the pelvic musculature and reproductive organs; (IV) resolution phase, with muscular relaxation and an overall sense of well-being. Cleary, Hegarty and McCarthy have very recently proposed a more comprehensive approach of sexual health (16), encompassing the dimensions of Sexual Self-Concept (sexual self-esteem, body image and sexual framing schema), Sexual Functioning (sexual response cycle) and Sexual Relationships (intimacy and communication).
From the conceptual standpoint, for a sexual problem to be considered a sexual dysfunction, according to the American Psychiatric Association (APA), it has to recur or persist in time and cause marked personal distress or interpersonal difficulty (14).
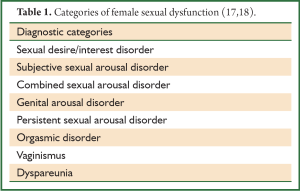
APA defined 5 categories: hypoactive sexual desire disorder (HSDD), female sexual arousal disorder (FSAD), female orgasmic disorder, dyspareunia and vaginismus (14). This classification was, nevertheless, based on the classical four-stage model of sexual response (15), and so it does not encompass the more subjective dimension of the female sexual experience (17). Consequently, a consensus panel organized by the International Committee of the American Foundation for Urological Disease (17,18) devised more comprehensive diagnostic criteria (Table 1). For diagnosis, other medical conditions or physiological drug-effects need to be ruled out (17,19) and the sexual problem needs to have a negative impact in the woman’s functioning, be it psychological or interpersonal (14,17).
The prevalence of each disorder is not accurate, mostly due to the fact that some disorders have only recently been identified (17,18) and accordingly lack evidence. As the majority of the studies used the APA classification, it is advised to consider the major categories of the disorders (desire/interest, arousal, orgasm, vaginismus/dyspareunia) when referring to them.
In the general adult female population, between 9-43% report having sexual problems (6,20), being the most commonly reported low desire (39%; causing distress in 10-14%), followed by low arousal (26%; causing distress in 5%) and orgasmic difficulties (21%; causing distress in 5%) (20). The reported rates of dyspareunia and vaginismus are about 16% (21,22).
Many of the studies conducted in BC patients were small, had sample biases, were retrospective or lacked a control group, but some suggest even that nearly all women present a problem in sexual functioning after BC treatment (3,6). The prevalence of the most commonly reported sexual problems in BC patients follows the aforementioned trend in the general population (4), generally with higher percentages, especially regarding dyspareunia and vaginismus (35-38%) (4). It is important to stress that most studies do not report the magnitude of sexual impairment before the cancer diagnosis, so part of the problem could already be pre-existent and hence not be fully explained by the oncologic experience (3,6).
Impact of the oncological diagnosis and treatment in the sexual health of young women with breast cancer
Change in the hormonal milieu
Abrupt menopause caused by cancer treatment, with the clustering of vasomotor symptoms, sleep disturbances, and vaginal dryness and atrophy, is more impairing and symptomatic than when following natural menopause (9). The transient or permanent ovarian failure induced by chemotherapy, hormonal therapy or ovarian suppression, causes depletion of the circulating level of estrogens and testosterone, two steroid hormones that play an important role in sexual functioning. Lack of estradiol is associated with diminished libido and sexual responsivity, hypoestrogenization impairs vulvovaginal vasocongestion during arousal and causes vulvovaginal atrophy and dryness which can lead to pain during intercourse (7,23,24). Studies evaluating the correlation between androgens [testosterone, androstenedione, dihydrotestosterone and dehydroepiandrosterone (DHEA)] and female sexual function have reported conflicting results (18,24,25). The fact testosterone is a precursor for estrogen formation renders difficult the distinction between the physiological effects of the two hormones (18); still, testosterone has vasodilatory effects, and may be linked to vaginal health and also an increase in libido and arousal, mainly in the postmenopausal population (26-28).
Breast cancer surgery
The association between the type of surgery, body image and sexual functioning has provided inconsistent results (3,10,29,30). One confounding factor in most studies (due to the multimodal treatment often necessary in young women with BC) might be chemotherapy, as women who received it do systematically worse in sexual functioning than women not having been submitted to it (3,9,11,25,29). The complexity also arises from the different “breast concepts” at play. Langellier and Sullivan identified four different, yet closelly related, “types of breasts” present on the speech and experience of BC patients (31): (I) the “medicalized breast”—the diseased body part, whose removal is usually accompanied by relief; (II) the “functional breast”—symbol of the nurturing quality of women, particular important in her relationship to her children; (III) the “gendered breast”—synonym of femininity, beauty and sexual attractiveness, on the personal and social spheres; (IV) the “sexualized breast”—pertaining to the tactile and visual sensations of the organ itself (30,31). It is, therefore, understandable that women can have dichotomous feelings about the breast and its changes during the cancer diagnosis and treatment. As an example, in some cultures and ethnic environments, undergoing a mastectomy and losing one breast is regarded as becoming “half a woman” (32).
Evidence shows that, overall, women with a better bodily self-image prior to the BC diagnosis cope better and have higher sexual satisfaction scores than women with a worse prior body image; the same holds true for women without and with previous mood disorders (anxiety, depression), respectively (25,29,30); also the HRQoL impact of the body alterations seems to be higher in the first year post-diagnosis, improving thereafter (30,33).
With respect to the type of surgical procedure, despite some controversial results (29,34,35), a growing body of evidence states that body image is significantly better in women who have undergone breast conserving surgery, as opposed to mastectomy, but this is the only aspect where it impacts sexuality (3,10,30). Indeed, giving the patient an active role in the choice of surgery, rather than the extent of the surgery itself, seems to be a major determinant of satisfaction with self-image; the empowerment of the woman on the preferences about her own body is far more crucial than the outcome itself (3,5,7).
In patients having undergone breast reconstruction, it is provoking to acknowledge that, although the final results are considered satisfactory by most, they report not having been properly informed about loss of nipple and breast sensation (4,36). Newer oncoplastic surgery techniques promise better overall cosmetic results (7).
There is no consensus regarding the impact on sexual functioning of the interval between surgery and the resumption of sexual intercourse (29) although some have found the longer the deferment, the higher the chance of sexual dysfunction (8).
Breast cancer systemic therapy
The alterations in sexual functioning brought about by antineoplastic drugs may be temporary or permanent and depend greatly on the drug class, total dose delivered, schedule of administration and time-length of therapy, as well as concomitant use of other antineoplastics or drugs that can modulate their action (37).
Globally, chemotherapy is a major determinant of sexual dysfunction, affecting all the phases of the sexual response cycle (30,38). This repercussion is particularly stern and catastrophic for young women, who are frequently also dealing with the grief of infertility (5,9,29,30). Cytotoxic chemotherapy, besides the chemotherapy-induced amenorrhea and ovarian failure entailing the endocrine consequences earlier described, also causes alopecia, nails changes and weight gain or loss (7,8,29,37,39), affecting women’s sexual self-concept and, consequently, their sexual interactions.
Primary ovarian failure is more common with alkylating agents, antimetabolites, vinca alkaloids, combination protocols and dose-dense regimens (37,39).
Drugs that act on the immune system, such as the targeted monoclonal antibodies trastuzumab and pertuzumab, or colony-stimulating factors may cause tiredness, flu-like symptoms and bone pain, decreasing sexual desire and altering body image (7). Other targeted agents, such tyrosine kinase inhibitors (lapatinib), mammalian target of rapamycin inhibitors (everolimus), or antiangiogenic agents (bevacizumab) have adverse effects such as fatigue, diarrhea, rash and hypertension, which, besides body-image transformation, can potentially lead to diminished interest in engaging in sexual activity and social isolation (7).
Hormonal treatments, such as antiestrogens, estrogen receptor antagonists, aromatase inhibitors (seldom used in premenopausal BC patients) and gonadotropin-releasing hormone (GnRH) agonists all have similar effects on sexual function (7,9), ultimately originating lack of genital lubrication and subsequent dyspareunia (nonetheless reversible after end of treatment), hot flashes, decreased interest in sex, weight gain and mood changes. Tamoxifen is an exception regarding vulvovaginal health, since its estrogenic effect in the vaginal epithelium may actually prevent vaginal dryness (7,9).
Comorbid conditions, concomitant medications and relationship factors
Mood disturbances, as anxiety and depression, are highly prevalent in BC patients, with depressive symptoms being particularly marked after diagnosis and during the active treatment phase (40). They have also shown to be strong correlates of sexual dysfunction, especially in the domains of low desire and anorgasmia, in several studies in both sexes and in cancer and non-cancer patients (20,25,41). In women submitted to chemotherapy as high as 30-40% can experience a severe and disabling degree of distress that can last for years following diagnosis (41). The psychotropic medications used in the treatment of these conditions have well recognized side effects on sexual function (7,41,42). This stems from their interference with neurotransmitters that act on the central modulation of sexual response: they inhibit dopamine and norepinephrine, which are involved in the arousal phase, and may also increase prolactin, eliciting gonadal suppression (18).
The most common drugs used to treat chemotherapy side effects, such as nausea and vomiting, also have anti-serotonin and anti-dopaminergic effects (there is wide pharmacological class overlap), and hence evoke similar consequences to antidepressants and anxiolytics (7,41,42). Beta-blockers, sometimes prescribed for anxiety, also have a detrimental impact on sexuality (41,43).
In partnered patients, the quality of their relationship is a critical and concordant predictor of sexual functioning (30), surpassing physical changes as a determinant for sexual health (8,30). The partner’s understanding and acceptance, a strong intimate bond and good communication and affection help in the sexual renegotiation process that follows the oncologic experience (7,8,30). Young women and their partners seem to need additional focus from healthcare providers, since young husbands are less skilled to cope with illness and, not rarely, the care of young children (8).
Assessment of sexual dysfunction in young women with breast cancer
The diagnostic assessment of patients combines the identification of the diagnostic criteria, elicited by conducting a thorough medical and sexual history, with a pelvic examination (the former is mandatory for sexual pain disorders, but should be performed in all patients with sexual complaints, because it can reveal possible loco-regional etiological factors and co-morbidities).
Presently, there is no gold standard for the evaluation of sexual health problems in cancer patients (4).
The initial step is to discuss the issue. Evidence shows that oncologists and ancillary care providers often feel reluctant to raise the subject, because of inadequate training in sexual matters, personal or patient awkwardness or lack of time (4,7). On the other hand, this leaves the patient uninformed, with unmet needs, conveying the message that sexual dysfunction is meaningless, or that it is a treatment side effect to which there are no solutions and that must be endured in silence (4,7). In spite of the communication barriers and mismatch in expectations between patients and providers, some data suggest patients are quite willing to debate the subject, even though they prefer the healthcare provider to initiate the discussion (7) and sometimes bring it up more easily with their general practitioner than their oncologist (5). Including sexual health as a part of the initial routine oncological treatment plan and follow-up would obviate many of these obstacles and should be an imperative in all practices providing breast cancer treatment (4,9).
One of the most widely accepted screening sexual models that could be useful in oncology is the PLISSIT model created by Annon (7,41): Permission (to discuss the subject), Limited Information (not to overwhelm the patient), Specific Suggestions (to-the-point pragmatic information) and Intensive Therapy (in the case of expert referral needed) (7,41). Concerning patient-reported outcomes, the most widely used questionnaire for women is the Female Sexual Function Index (FSFI), a 19-item instrument covering 6 domains of sexual functioning: sexual desire, sexual arousal, lubrication, orgasm, satisfaction and pain (44). The provider should devoid his speech of technical jargon and colloquialisms, avoid cultural and ethnical stereotyping and also avert being judgmental (7); this is particularly true regarding singled patients, whose sexuality is often dismissed by the provider, and that may have or be attempting sexual interactions and have questions and needs that require equal addressing (4).
Management of sexual dysfunction in young women with breast cancer
There is a dearth for evidence-based treatments for sexual dysfunction in the context of breast cancer, even more so for young patients, due to the lack of attention received by this subgroup in the majority of studies conducted thus far (4).
Setting realistic tailored treatment goals, using a multidisciplinary team approach (oncologist, nurse, psychologist, psychiatrist, sex therapist, pelvic physical therapist) and treating associated conditions that might be at the origin of the problem (for e.g., changing an antidepressant by another of a different class with a better profile regarding sexual side effects) should be principles of intervention. In partnered relationships the partners should be involved in the intervention, by some form of couple’s therapy, which has been proven to be one of the most effective non-pharmacological strategies (4,45). Brief counseling or short-term sex therapy programs can yield positive results (4). For younger patients, community-based support, retreats and social programs look more appealing and suitable (7).
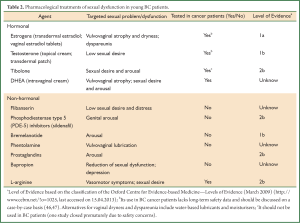
Pharmacological treatments are summarized in Table 2 (4,9,18,48).
Full Table
Future directions: an integrative approach for the improvement of sexual health management in breast cancer oncology practice
Whereas BC treatment does not radically differ according to the patients’ age group, ideal management of BC patients below 40 years of age demands attention to certain issues that are specific of that age group (8,49) and, thereupon, certain resources that may not be widely available in oncology clinics. As a proof, one can look at the low rates of compliance to the American Society of Clinical Oncology guidelines pertaining to the aspects of discussion of infertility and proper referral to reproductive specialists before start of therapy (50). Dedicated holistic programs such as PYNK (49), involving a multidisciplinary committee, and joining patient psychosocial support, gatherings, a sexual health and rehabilitation clinic with research and educational efforts, are in urgent need of dissemination (49). Meanwhile, oncologic clinics should pay greater attention to patients’ sexual health and, in the event of not being endowed with the appropriate resources, provide external referral (4,5). Ethnic diversity with differing sexual constructs and linguistic factors also needs examination, notably in a growing multicultural society (4). Sexual functioning in the cancer continuum is a topic of increasing relevance and it claims competent addressing in the growing communities of younger patients and cancer survivors.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44. [PubMed]
- Sadovsky R, Basson R, Krychman M, et al. Cancer and sexual problems. J Sex Med 2010;7:349-73. [PubMed]
- Ganz PA. Sexual functioning after breast cancer: a conceptual framework for future studies. Ann Oncol 1997;8:105-7. [PubMed]
- Bober SL, Varela VS. Sexuality in adult cancer survivors: challenges and intervention. J Clin Oncol 2012;30:3712-9. [PubMed]
- Kedde H, van de Wiel HB, Weijmar Schultz WC, et al. Sexual dysfunction in young women with breast cancer. Support Care Cancer 2013;21:271-80. [PubMed]
- Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med 2011;8:294-302. [PubMed]
- Krebs LU. Sexual health during cancer treatment. Adv Exp Med Biol 2012;732:61-76. [PubMed]
- Cardoso F, Loibl S, Pagani O, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer 2012;48:3355-77. [PubMed]
- Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol 2008;26:753-8. [PubMed]
- Lam WW, Li WW, Bonanno GA, et al. Trajectories of body image and sexuality during the first year following diagnosis of breast cancer and their relationship to 6 years psychosocial outcomes. Breast Cancer Res Treat 2012;131:957-67. [PubMed]
- Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst 2002;94:39-49. [PubMed]
- Odo R, Potter C. Understanding the needs of young adult cancer survivors: a clinical perspective. Oncology 2009;23:23-7, 33. [PubMed]
- Organization WH. What constitutes sexual health? 2010.
- Association AP. eds. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition - Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association editions, 2000.
- Masters W, Johnson V. eds. Human sexual response. Toronto, New York: Bantam Books, 1966.
- Wylie K, Mimoun S. Sexual response models in women. Maturitas 2009;63:112-5. [PubMed]
- Basson R. Women’s sexual dysfunction: revised and expanded definitions. CMAJ 2005;172:1327-33. [PubMed]
- Fooladi E, Davis SR. An update on the pharmacological management of female sexual dysfunction. Expert Opin Pharmacother 2012;13:2131-42. [PubMed]
- Basson R, Leiblum S, Brotto L, et al. Definitions of women’s sexual dysfunction reconsidered: advocating expansion and revision. J Psychosom Obstet Gynaecol 2003;24:221-9. [PubMed]
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol 2008;112:970-8. [PubMed]
- Pitts MK, Ferris JA, Smith AM, et al. Prevalence and correlates of three types of pelvic pain in a nationally representative sample of Australian women. Med J Aust 2008;189:138-43. [PubMed]
- Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc 2003;58:82-8. [PubMed]
- Shafer L. Sexual dysfunction. In: Carlson K, Eisenstat S. eds. Primary care of women. St. Louis, MO: Mosby, 2002:415.
- Dennerstein L, Dudley EC, Hopper JL, et al. Sexuality, hormones and the menopausal transition. Maturitas 1997;26:83-93. [PubMed]
- Speer JJ, Hillenberg B, Sugrue DP, et al. Study of sexual functioning determinants in breast cancer survivors. Breast J 2005;11:440-7. [PubMed]
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 2000;343:682-8. [PubMed]
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab 2005;90:5226-33. [PubMed]
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause 2006;13:770-9. [PubMed]
- Fobair P, Stewart SL, Chang S, et al. Body image and sexual problems in young women with breast cancer. Psychooncology 2006;15:579-94. [PubMed]
- Gilbert E, Ussher JM, Perz J. Sexuality after breast cancer: a review. Maturitas 2010;66:397-407. [PubMed]
- Langellier KM, Sullivan CF. Breast talk in breast cancer narratives. Qual Health Res 1998;8:76-94. [PubMed]
- Manderson L, Stirling L. The absent breast: speaking of the mastectomised body. Feminism Psychol 2007;17:75-92.
- Joly F, Espie M, Marty M, et al. Long-term quality of life in premenopausal women with node-negative localized breast cancer treated with or without adjuvant chemotherapy. Br J Cancer 2000;83:577-82. [PubMed]
- Parker PA, Youssef A, Walker S, et al. Short-term and long-term psychosocial adjustment and quality of life in women undergoing different surgical procedures for breast cancer. Ann Surg Oncol 2007;14:3078-89. [PubMed]
- Han J, Grothuesmann D, Neises M, et al. Quality of life and satisfaction after breast cancer operation. Arch Gynecol Obstet 2010;282:75-82. [PubMed]
- Snell L, McCarthy C, Klassen A, et al. Clarifying the expectations of patients undergoing implant breast reconstruction: a qualitative study. Plast Reconstr Surg 2010;126:1825-30. [PubMed]
- Azim HA Jr., Peccatori FA, de Azambuja E, et al. Motherhood after breast cancer: searching for la dolce vita. Expert Rev Anticancer Ther 2011;11:287-98. [PubMed]
- Ochsenkühn R, Hermelink K, Clayton AH, et al. Menopausal status in breast cancer patients with past chemotherapy determines long-term hypoactive sexual desire disorder. J Sex Med 2011;8:1486-94. [PubMed]
- Rodriguez-Wallberg KA, Oktay K. Options on fertility preservation in female cancer patients. Cancer Treat Rev 2012;38:354-61. [PubMed]
- Pinto AC, de Azambuja E. Improving quality of life after breast cancer: dealing with symptoms. Maturitas 2011;70:343-8. [PubMed]
- Dizon DS. Quality of life after breast cancer: survivorship and sexuality. Breast J 2009;15:500-4. [PubMed]
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet 2007;369:512-25. [PubMed]
- Addis IB, Ireland CC, Vittinghoff E, et al. Sexual activity and function in postmenopausal women with heart disease. Obstet Gynecol 2005;106:121-7. [PubMed]
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191-208. [PubMed]
- Taylor S, Harley C, Ziegler L, et al. Interventions for sexual problems following treatment for breast cancer: a systematic review. Breast Cancer Res Treat 2011;130:711-24. [PubMed]
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab 2010;95:s1-s66. [PubMed]
- Davis SR, Moreau M, Kroll R, et al. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med 2008;359:2005-17. [PubMed]
- Shifren JL. Sexual dysfunction in women: Management. In: Rose BD. eds. Waltham, UpToDate in Waltham, MA, 2013.
- Ali A, Warner E. pynk: Breast Cancer Program for Young Women. Curr Oncol 2013;20:e34-9. [PubMed]
- Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol 2009;27:5952-7. [PubMed]