Epidemiology and prognosis of breast cancer in young women
Introduction
Breast cancer is the most common non-cutaneous malignancy, accounting for nearly one in three cancers diagnosed among women in the United States, and the second leading cause of cancer death around the world (1,2). Around 6.6% of all breast cancer cases are diagnosed in women less than 40 of age, 2.4% in women less than 35, and 0.65% in women less than 30 (3,4); if plotted on a curve, the cumulative incidence of breast cancer seems to follows an exponential function below the age of 40 after which it seems to rise linearly (3). The overall worldwide burden of breast cancer has doubled between 1975 and 2000, and this is thought to be attributable to the increasing life expectancy and widespread adoption of westernized lifestyle with all its risk factors (5). However, these trends are not seen in early onset breast cancer, as the rates have been more or less stable in most countries in the past 20 years (6). As for death rates, they have been steadily decreasing, especially in younger women, owing to the improved treatment and early detection (7); however, breast cancer in young women remains a great challenge to patients, families and health care providers. Although the diagnosis of breast cancer is much less common in women under the age of 40 years, it can have a greater impact than in older counterparts, as it tends to present at a later stage, be more aggressive and have a poorer prognosis (8,9).
Many studies have suggested that age is an independent prognostic factor; however, this issue is now considered controversial. Breast cancer in young women is more likely to be of a more aggressive subtype, such as triple negative or HER2-positive breast cancer, and is more likely to present at an advanced stage, either because of its biological aggressive subtype or because of a low index of suspicion and delayed diagnosis. This may translate into more loco-regional recurrences and distant metastases, which contributes to the poorer outcome of young women with breast cancer.
In this article, we will review epidemiology and differences between various populations and regions of the world, as well as prognosis and outcome of young women with breast cancer.
Epidemiology
According to GLOBOCAN-generated data of 2008, more than 146,660 new cases of breast cancer have been diagnosed in women less than 40 years of age worldwide, with an age-standardized rate per 100,000 (ASR) of 6 (10). Early onset breast cancer trends vary among populations and areas of the world. Although 77% of the cases occurred in developing countries, the ASR for women below the age of 40 was marginally higher in developed countries (8.8 vs. 5.4) (10). Overall, GLOBOCAN-generated rates of breast cancer in women less than 40 years in different countries have shown relatively stable annual rates around the world, ranging from an ASR of 1.1 to 17. This is in contrast to the overall breast cancer population rates, which vary from 8 to 109 (10). The lowest rates come from countries in Eastern and Southern Africa, while the highest rates are recorded in Europe and North America. Rates of breast cancer below and above 40 years from selected countries are presented in Table 1. These differences are less likely related to screening practices, since screening recommendation is not offered before the age of 40, nor to the use of HRT, since patients are premenopausal (6). It is important to note that not all countries have sufficient data and statistics on cancer rates. Most data come from high-income industrialized countries and tend to be the most accurate, precise, and up-to-date. In the USA, the Surveillance, Epidemiology and End Results (SEER) program is a principal source for cancer statistics in the country, and extensive analysis of these data are periodically published in the literature. Pertaining to our topic, SEER data between 1988 and 2003 showed an incidence of breast cancer below the age of 40 of 6.4% (15,548 patients) out of the total breast cancer population in that period (243,012 patients) (11). In addition to published data from many countries, GLOBOCAN includes other countries with lacking data by making extrapolations of old statistics or nearby population statistics (1).
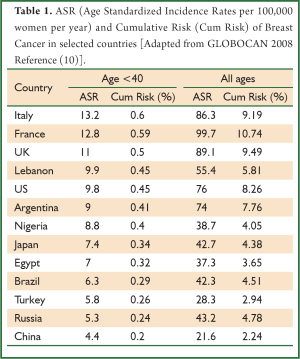
Full Table
Risk factors
Differences exist between populations that are not predictive of early onset breast cancer, such as fertility rates, which vary from 1.4 in Japan, to 2.1 in USA, to 5.3 in Nigeria, to 2.9 in Egypt (12). These countries have close cumulative risk rates of early onset breast cancer (0.34 in Japan, 0.45 in USA, 0.4 in Nigeria, and 0.32 in Egypt) despite varying fertility rates (10). Early onset breast cancer does not seem to be directly related to westernization or standard of living, where a weak correlation is found between country income level and early onset breast cancer (6). Genetic factors may play a role in affecting rates of early onset breast cancer in different areas, though their role cannot by itself account for international variation in risk. In the UK, approximately 3% of all breast cancers are attributable to mutations in BRCA1 or BRCA2 (13), whereas this number increases in Ashkenazi Jews to up to 40% (14). TP53 mutation, although vary rare, is the causative agent of breast cancer in Li-Fraumeni syndrome, which tends to affect women between 20 and 40 years of age (15). Some populations such as in Southern Brazil have relatively high mutation frequency of TP53 mutation, reaching one in 300 women (16,17). Hormonal factors also vary in different populations, races, and ethnicities. In a study by Lund et al. (18), in Atlanta, USA, incidence rates of triple-negative tumors differed by race, with an incidence of 36.3 per 100,000 for black women, and 19.4 per 100,000 for white women.
Environmental factors
Nevertheless, most of the variation in risk is believed to be due to differential environmental exposure to certain risk factors. Studies of migrants further emphasize this hypothesis; incidence of cancers tend to rise following migration from low to high incidence countries, especially if it occurs early in life (19). Many risk factors for breast cancer have been well-established by case-control and cohort studies. However, there have been few efforts to quantify the magnitude of risk disparities between countries that might be explained by such factors.
The role of risk factors in early-onset breast cancer is even less clear. Studies involving this category of breast cancer patients are usually hindered by small sample sizes (6). Moreover, factors such as intrauterine exposures would be of utmost difficulty to follow in cohort studies. Nevertheless, case-control studies have shown that birth weight, growth rate in childhood, and attained height are all risk factors for premenopausal breast cancer (20,21). It has been postulated that prenatal influences, including hormones and growth factors, may alter the risk of breast cancer, but such correlations would be very difficult to measure (22).
Exercise, diet and obesity
Although many studies showed a favorable outcome of exercise regarding breast cancer risk (23), some studies failed to show it (24,25). In a prospective study of 64,777 premenopausal women, physical activity was associated with a 23% breast cancer risk reduction. However, in a prospective study involving 218,169 subjects in 9 European countries published in 2007, exercise was not found to be associated with early breast cancer risk (25). Overall, a systematic review of 76 studies on this topic reported that 53% of studies confirmed a significant protective effect, 37% reported a non-significant risk reduction, and only 10% failed to show a correlation (26). With regards to diet, results of observational studies were inconsistent. While several observational studies of fruit and vegetable consumption did not show any benefit in reducing breast cancer risk in both premenopausal and postmenopausal women (27), the Nurses’ Health study showed nearly a 50% greater risk of breast cancer in premenopausal women who consume a high animal fat diet, but not with women on high vegetable fat diet (28). Moreover, experimental and human data shows that Mediterranean diet rich in extra virgin oil is associated with reduced risks of breast cancer (29). As for the effect of high BMI, it seems to have opposite effects in pre- and postmenopausal women. Obesity is known to increase the risk of breast cancer in postmenopausal women, probably due to the increase in estrogen exposure caused by aromatization in fatty tissues (30). In contrast, a high BMI seems to be protective in the premenopausal group (30), for reasons which are still unknown.
Female hormone exposure
In an analysis done by the Collaborative Group on Hormonal Factors in Breast Cancer (31) on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 studies conducted in 25 countries, the risk of breast cancer was increased in current oral contraceptive users as well as women within 10 years of OCP stoppage. However, the increased risk is very small, being the greatest at 1.24 (95% CI, 1.15-1.33) in current OCP users (31). Regarding other reproductive risk factors, a review of 26 articles showed various degrees of effect on breast cancer risks (32). For each additional year at menarche, a decrease of about 9% of breast cancer risk was found in premenopausal women, and of about 4% when diagnosed at older age (32). Breast cancer risk increased with increasing age at first full term pregnancy (FFTP) by 5% per year for breast cancer diagnosed before menopause versus 3% for breast cancers diagnosed after menopause (32). There was a 3% reduction in premenopausal breast cancer risk for each full term pregnancy, whereas the reduction reached 12% in postmenopausal women (32). Every 12 months of breast-feeding decreased the risk by 4.3% in both premenopausal and postmenopausal women (33).
Genetic mutations
In addition to the above risk factors, it has been postulated that the set of molecular and genetic characteristics of breast carcinomas that arise in younger women, such as BRCA mutations, may be different from that of older women, much like the variance between different populations described above. In Britain, the proportion of breast cancer patients with BRCA1 or BRCA2 mutations is higher in women less than 36 years of age compared to the whole breast cancer population group (6% vs. 3%) (13,34).
Prognosis
Based on various prospective and retrospective studies performed in the last two decades, it has been generally accepted that young age at diagnosis correlates with a worse clinical outcome compared to their older counterparts (3,35-40). This holds true irrespective of menopausal status, as age is still a risk factor among premenopausal women (41). In addition, breast cancer survival rates are comparatively lower for women less than 40 years of age than for older women across all histological subtypes and stages (3). However, the controversy lies in the question of whether age per se is an independent risk factor for worse prognosis. Many studies have refuted this hypothesis; they rather propose that the effect of young age on outcome is merely a reflection of over-representation of other known prognostic pathological factors, such as higher grade of differentiation, presence of lymphovascular invasion, higher mitotic rate, lower ER/PR expression, and higher HER2 expression (42-45). Yet other studies have attributed the inferior outcome of young age to the more advanced presentation at diagnosis, including higher rates of axillary lymph node positivity and larger tumor size (39,46-48). Others have postulated that the effect of differential gene expression between different age groups might play a role (49,50). In any case, knowing the true impact of age on prognosis may have an effect on our management. If it is indeed an independent factor, then young women might benefit from more aggressive treatment than their older counterparts with the same clinical and pathological scenario.
Breast cancer subtypes
It is well established that there are at least 4 main subtypes of breast cancer based on different patterns of gene expression, and that they have a considerable impact on prognosis (51,52). Luminal A includes ER+ and/or PR+ and HER2-, grade 1 or 2 tumors, and they tend to have the best prognosis (52). Luminal B comprises ER+ and/or PR+ and HER2+, or ER+ and/or PR+ and HER2-, grade 3 tumors. The other 2 subtypes are the HER2 overexpressing tumors (ER-, PR-, HER2+) and the triple negative tumors (ER-, PR-, HER-), both of which confer bad prognosis (51). Many studies have confirmed the increased proportion of ER/PR-negativity, HER2 overexpression, and high grade in young women with breast cancer (50,53). Therefore, this could partly account for the worse outcome of young age. A study of 399 breast cancer patients below 40 years by Collins et al. (53) revealed a lower proportion of luminal A disease (33% vs. 60-70%) and higher proportion of luminal B disease (35% vs. 6-22%) compared to numbers from population studies of breast cancer (53-57). Fifty-five percent of patients had high grade tumors, and 31% of all tumors overexpressed HER2 (53), which is high compared to the 12.6% presented in a study of 1,842 breast cancer patients in Atlanta by Lund et al. (18). Triple negative tumors have also been found to be over-represented in young women with breast cancer, with rates close to 26% (58). To further confirm the above clinical findings, Anders et al. (50) studied the mRNA expression of ERα, ERβ, PR, and HER2 in 200 patients ≤45 years and 211 patients ≥65 years. Young patients had a lower expression of ERα (7.2 vs. 9.8, respectively; P=0.0001), ERβ (5.6 vs. 5.9, respectively; P=0.02), and PR (4.1 vs. 5.0, respectively; P=0.001) compared to their older counterparts. As for HER2 expression, it was statistically higher in the age group ≤45 years compared to the age group ≥65 years (11.1 vs. 9.4, respectively; P=0.0001).
Advanced stage at presentation
Several studies raised the notion that young breast cancer patients tend to present with more advanced stages than older women (39,46-48). A retrospective cohort from Denmark of 10,356 women diagnosed before 50 years reported that patients aged ≤35 years at diagnosis were at higher risk of being node positive (51% vs. 46%; P=0.02) compared with patients between 35 and 50 years (47). A study of 732 non-metastatic breast cancer patients from Mount Sinai Medical Center, New York showed that patients younger than 36 years had larger tumors (median 2.0 vs. 1.5 cm, Pvs. 37%, P=0.022), and were more likely to be diagnosed with stage II or III cancer (60% vs. 43%, P48).
Genetic mutations, gene microarrays
Another reason for young patients presenting with more aggressive tumors is the higher proportion of BRCA1 and BRCA2 mutations (34,59), which are known to be associated with higher histological grade, higher proliferation rate and ER negativity (60). Other genetic variances have also been studied. According to Dubsky et al. (41), p53 mutation, c-erbB-2 over expression and tumor proliferation markers are associated with a young age and an increase in local recurrence probability, and thus more aggressive tumors. In 2008, Andres et al. (50) identified 367 gene sets that may make a distinction between breast tumors in young women from those in older women, which may have an impact on prognosis. Moreover, a recent study by Azim et al. (49) assessed the differential role of proliferation, stroma, and immune-related gene signatures in providing prognostic information in different breast cancer subtypes. They further confirmed the age-dependent differential expression of genes associated with immature mammary cell populations (RANKL, c-kit, BRCA1-mutated phenotype, mammary stem cells, and luminal progenitors cells), and growth factor signaling [mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)-related].
Based on the aforementioned adverse pathological and possible genetic characteristics present in young breast cancer patients, one could explain the poor outcome in this patient population. However, many studies showed that even after accounting for these factors, young age per se seems to act independently in affecting prognosis.
Risks of local recurrence after primary therapy
Risk of local recurrence of breast cancer has been shown to be increased in young patients in two separate analyses of clinical trials, namely the EORTC group trials and NSABP group trials (61,62). The former showed a hazard ratio of 2.8 (95% CI, 1.4-5.6) for local recurrence in patients less than 35 years compared to those above 50 years. A study by Bharat et al. (36) estimated the risk of breast cancer recurrence for women diagnosed below the age of 40 to be 1.53 (95% CI, 1.37-1.74) times higher than in those diagnosed above 40 years. Voogd et al. (63) combined data on stage I and II breast cancer patients from 2 large clinical trials (EORTC and DBCG). They reported a 9.2-fold (95% CI, 3.7-23) higher risk of local recurrence in women aged 35 who underwent breast conserving surgery compared to women 65 years and above. As for distant recurrence, the risk was doubled (95% CI, 1.26-3.96) in the young patient group compared to the older patients (63). If we look at studies examining rates of contralateral breast cancer (CBC) risks, we can deduce that young age is a quite a strong risk factor (64,65). Although the absolute risk of CBC is similar between different age groups, the relative risk increase in younger populations is quite substantial, since the initial risk of breast cancer in young women is low compared their older counterparts (6,64).
Survival
Young age has also been shown to negatively affect survival. A large prospective cohort of 2,956 breast cancer patients less than 40 years diagnosed between 2000-2008 in 126 UK hospitals reported a 5-year overall survival of 82% (66). This is relatively low considering the fact that only 2.5% of patients had metastasis at presentation. Many studies compared data from young women to their older counterparts. Data from 1,398 patients analyzed by Nixon et al. (67) showed that young age (vs. >35) remained an important predictor of mortality after adjusting for confounding variables, with a relative risk of 1.50. At the Institut Curie in France, it was shown that even after adjusting for clinical tumor size, node status, histological grade, hormone receptor, locoregional treatment procedure and adjuvant systemic therapy, both overall survival and disease-free survival continued to be lower in the younger age group (38). Another study by Gnerlich et al. (11) reviewed 243,012 breast cancer patients in the SEER database from 1988-2003. Young women less than 40 years had a higher breast cancer mortality rate (18.3% vs. 12.1%, P=0.001) than those older than 40 years. If adjusted for other prognostic factors and stratify by stage, younger women were more likely to die from breast cancer compared with older women if diagnosed with stage I (HR=1.44; 95% CI, 1.27 to 1.64) or stage II (HR=1.09; 95% CI, 1.03 to 1.15) disease; however, age lost its prognostic value in more advanced disease (11). A similar trend was also seen in a study of 185 patients less than 30 years in MD Anderson Cancer Center (68).
Conclusions
Age at diagnosis remains an important factor for prognostication and treatment decisions in patients with breast cancer. Although, breast cancer in women below 40 years of age constitutes a small proportion of the total incidence, it has a significant burden on women and society. Incidence rates and cumulative risk rates in women below 40 years vary little between populations, but generally remain low and do not justify screening in average risk women. Risk factors in breast cancer do not necessarily have the same effect in young and older patients. While a high BMI seems to have a protective effect against development of breast cancer in premenopausal women, controversy still surrounds the influence of diet and physical activity in this population. Breast cancer in young women is associated with a poorer outcome, partly because of over-representation of more aggressive subtypes, such as triple negative or HER2-positive breast cancer. In addition, they are more likely to present at an advanced stage or have a delayed diagnosis because of a low index of suspicion by the patient and the primary physician. These factors predispose to more loco-regional recurrences and distant metastases which contribute to the poorer outcome of young women with breast cancer. Many studies have shown a worse prognosis even after controlling for pathological factors and staging. However, the discovery of more prognostic markers and factors might weaken the correlation between age and outcome. Management of young women with breast cancer still requires particular attention to surgical negative margins, long term follow-up after breast-conserving therapy, more aggressive adjuvant therapy because of poorly differentiated histologies, receptor negative and/or HER2-positive tumors, or poor gene signatures, and to improvement of access to care worldwide.
Review criteria
The articles that were reviewed for this manuscript were based on a keyword search using PubMed 1975 to present (April 2013), searching the title containing the word string “breast cancer” AND (“young” OR “age”). Only articles published in English were considered. All titles were reviewed but only those articles that were considered to be the most relevant to our topic were included in the review. Additional pertinent articles were included if these were deemed to be relevant by the author.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- DeSantis C, Siegel R, Bandi P, et al. Breast cancer statistics, 2011. CA Cancer J Clin 2011;61:409-18. [PubMed]
- Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol 2009;36:237-49. [PubMed]
- Fredholm H, Eaker S, Frisell J, et al. Breast cancer in young women: poor survival despite intensive treatment. PLoS One 2009;4:e7695. [PubMed]
- Boyle P, Howell A. The globalisation of breast cancer. Breast Cancer Res 2010;12 Suppl 4:S7. [PubMed]
- Narod SA. Breast cancer in young women. Nat Rev Clin Oncol 2012;9:460-70. [PubMed]
- Ferguson NL, Bell J, Heidel R, et al. Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women. Breast J 2013;19:22-30. [PubMed]
- Han W, Kim SW, Park IA, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer 2004;4:82. [PubMed]
- Brennan M, French J, Houssami N, et al. Breast cancer in young women. Aust Fam Physician 2005;34:851-5. [PubMed]
- Ferlay J, Shin H, Bray F, et al. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available online: http://globocan.iarc.fr, Accessed April 5, 2013.
- Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg 2009;208:341-7. [PubMed]
- The World Factbook 2013-14. Washington, DC: Central Intelligence Agency, 2013. Available online: https://www.cia.gov/library/publications/the-world-factbook/index.html, Accessed March 18, 2013.
- Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer 2000;83:1301-8. [PubMed]
- Warner E, Foulkes W, Goodwin P, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 1999;91:1241-7. [PubMed]
- Nichols KE, Malkin D, Garber JE, et al. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol Biomarkers Prev 2001;10:83-7. [PubMed]
- Palmero EI, Schüler-Faccini L, Caleffi M, et al. Detection of R337H, a germline TP53 mutation predisposing to multiple cancers, in asymptomatic women participating in a breast cancer screening program in Southern Brazil. Cancer Lett 2008;261:21-5. [PubMed]
- Gomes MC, Kotsopoulos J, de Almeida GL, et al. The R337H mutation in TP53 and breast cancer in Brazil. Hered Cancer Clin Pract 2012;10:3. [PubMed]
- Lund MJ, Butler EN, Hair BY, et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes: a population-based study and first report. Cancer 2010;116:2549-59. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Ahlgren M, Sørensen T, Wohlfahrt J, et al. Birth weight and risk of breast cancer in a cohort of 106,504 women. Int J Cancer 2003;107:997-1000. [PubMed]
- Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and the risk of breast cancer in women. N Engl J Med 2004;351:1619-26. [PubMed]
- Trichopoulos D, Adami HO, Ekbom A, et al. Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer 2008;122:481-5. [PubMed]
- Maruti SS, Willett WC, Feskanich D, et al. A prospective study of age-specific physical activity and premenopausal breast cancer. J Natl Cancer Inst 2008;100:728-37. [PubMed]
- Lynch BM, Courneya KS, Friedenreich CM. A case-control study of lifetime occupational sitting and likelihood of breast cancer. Cancer Causes Control 2013;24:1257-62. [PubMed]
- Lahmann PH, Friedenreich C, Schuit AJ, et al. Physical activity and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 2007;16:36-42. [PubMed]
- Loprinzi PD, Cardinal BJ, Winters-Stone K, et al. Physical activity and the risk of breast cancer recurrence: a literature review. Oncol Nurs Forum 2012;39:269-74. [PubMed]
- van Gils CH, Peeters PH, Bueno-de-Mesquita HB, et al. Consumption of vegetables and fruits and risk of breast cancer. JAMA 2005;293:183-93. [PubMed]
- Cho E, Spiegelman D, Hunter DJ, et al. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst 2003;95:1079-85. [PubMed]
- Escrich E, Moral R, Solanas M. Olive oil, an essential component of the Mediterranean diet, and breast cancer. Public Health Nutr 2011;14:2323-32. [PubMed]
- Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast 2004;13:85-92. [PubMed]
- Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 1996;347:1713-27. [PubMed]
- Clavel-Chapelon F, Gerber M. Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat 2002;72:107-15. [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002;360:187-95. [PubMed]
- Peto J, Collins N, Barfoot R, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 1999;91:943-9. [PubMed]
- El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer 2006;6:194. [PubMed]
- Bharat A, Aft RL, Gao F, et al. Patient and tumor characteristics associated with increased mortality in young women (PubMed]
- Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol 2011;29:3885-91. [PubMed]
- de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet 1993;341:1039-43. [PubMed]
- Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer 1996;78:1838-43. [PubMed]
- Adami HO, Malker B, Holmberg L, et al. The relation between survival and age at diagnosis in breast cancer. N Engl J Med 1986;315:559-63. [PubMed]
- Dubsky PC, Gnant MF, Taucher S, et al. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer 2002;3:65-72. [PubMed]
- Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 2011;29:e18-20. [PubMed]
- Crowe JP Jr, Gordon NH, Shenk RR, et al. Age does not predict breast cancer outcome. Arch Surg 1994;129:483-7; discussion 487-8. [PubMed]
- Ezzat A, Raja MA, Zwaan F, et al. The lack of age as a significant prognostic factor in non-metastatic breast cancer. Eur J Surg Oncol 1998;24:23-7. [PubMed]
- Figueiredo JC, Ennis M, Knight JA, et al. Influence of young age at diagnosis and family history of breast or ovarian cancer on breast cancer outcomes in a population-based cohort study. Breast Cancer Res Treat 2007;105:69-80. [PubMed]
- Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr 1994;35-42. [PubMed]
- Kroman N, Jensen MB, Wohlfahrt J, et al. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ 2000;320:474-8. [PubMed]
- Gajdos C, Tartter PI, Bleiweiss IJ, et al. Stage 0 to stage III breast cancer in young women. J Am Coll Surg 2000;190:523-9. [PubMed]
- Azim HA Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 2012;18:1341-51. [PubMed]
- Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324-30. [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [PubMed]
- Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A 2003;100:10393-8. [PubMed]
- Collins LC, Marotti JD, Gelber S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat 2012;131:1061-6. [PubMed]
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [PubMed]
- Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 2008;10:R67. [PubMed]
- Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev 2007;16:439-43. [PubMed]
- Caldarella A, Crocetti E, Bianchi S, et al. Female breast cancer status according to ER, PR and HER2 expression: a population based analysis. Pathol Oncol Res 2011;17:753-8. [PubMed]
- Carvalho FM, Bacchi LM, Santos PP, et al. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics (Sao Paulo) 2010;65:1033-6. [PubMed]
- Malone KE, Daling JR, Neal C, et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer 2000;88:1393-402. [PubMed]
- Colleoni M, Rotmensz N, Robertson C, et al. Very young women (PubMed]
- de Bock GH, van der Hage JA, Putter H, et al. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer 2006;42:351-6. [PubMed]
- Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol 2006;24:2028-37. [PubMed]
- Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol 2001;19:1688-97. [PubMed]
- Hartman M, Czene K, Reilly M, et al. Genetic implications of bilateral breast cancer: a population based cohort study. Lancet Oncol 2005;6:377-82. [PubMed]
- Kollias J, Ellis IO, Elston CW, et al. Clinical and histological predictors of contralateral breast cancer. Eur J Surg Oncol 1999;25:584-9. [PubMed]
- Eccles B, Copson E, Maishman T, et al. Breast cancer diagnosis and treatment in women aged 18-40 years in the UK: Prospective study of Outcomes in Sporadic versus Hereditary breast cancer (POSH). Liverpool, UK: In: 8th NCRI Cancer Conference, 2012.
- Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol 1994;12:888-94. [PubMed]
- Xiong Q, Valero V, Kau V, et al. Female patients with breast carcinoma age 30 years and younger have a poor prognosis: the M.D. Anderson Cancer Center experience. Cancer 2001;92:2523-8. [PubMed]


