Comparison of fluticasone propionate with budesonide administered via nebulizer: a randomized controlled trial in patients with severe persistent asthma
Introduction
Asthma is a heterogeneous disease characterized by chronic airway inflammation, with various respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough, together with variable expiratory airflow limitation (1). Asthma is a common chronic disease affecting between 1% and 21.5% of the adult population globally (2). The prevalence in China is low relative to the global rate: the estimated rate of clinical (treated) asthma in China is 1.42%, while the equivalent global average is 4.46% (2). More recent data estimate the prevalence of asthma in China in those > 14 years as 1.24% (3).
Asthma is considered well managed with the achievement of good symptom control and minimal future risk of exacerbations, fixed airflow limitation and treatment-related adverse events (AEs) (1). Inhaled corticosteroids (ICSs) are the preferred treatment for controlling asthma symptoms, which are often used together with an as-needed short-acting beta agonist for quick relief of asthma symptoms (1). Treatment with low-dose ICS reduces asthma symptoms, increases lung function and reduces the risk of asthma-related death (4-6). Clinical management guidelines recommend a step-wise approach with increasing ICS dosage in the case of uncontrolled symptoms, exacerbations, or risks (1).
The efficacy and safety of fluticasone propionate (FP) inhalation solution is well established (4,6), and inhaled formulations are registered for the treatment of asthma in >140 countries worldwide, with post-marketing exposure cumulative to June 2013 estimated to be 41.4 million patient treatment years.
A systematic review and meta-analysis of 71 randomized studies comparing fluticasone with either beclomethasone (BDP) or budesonide (BUD) for the treatment of chronic asthma found that, at a dose ratio of 1:2, FP was at least as effective as the comparators in improving clinical outcomes such as FEV1 and exacerbations, and could be more effective than BDP or BUD in improving morning peak expiratory flow (PEF) (4).
ICSs, including FP, are available with different delivery devices, believed to result in differences in lung deposition properties, in vivo dosage accuracy, and dose variability (7). However, a recent study that compared the efficacy of fluticasone propionate administered with different delivery devices (dry powder inhaler, metered dose inhaler, metered dose inhaler with spacer and nebulizer) indicated that there was a similar effect on lung function in patients with chronic stable bronchial asthma, irrespective of delivery method (7).
In the inhaler presentation (pressurized metered dose inhalers and dry powder inhalers), FP can prove ineffective in some children, elderly asthma patients, and some patients with severe asthma, particularly those who experience difficulty with co-ordination or during exacerbations (8). In such instances, patients may be unable to generate the inspiratory flow required to use an inhaler effectively. Nebulizers, which convert liquid medication to a fine mist for inhalation, enable a high dose of FP to be delivered directly to the lungs, requiring the patient to use tidal breathing only.
FP at half the dose of BUD has been shown to be as, or more, effective for the treatment of asthma, in terms of morning PEF, compared with BUD, irrespective of delivery system (4,6). Findings regarding the comparative safety and efficacy of FP solution for inhalation have not previously been confirmed in Chinese patients with severe, persistent asthma. This study was designed to assess the safety and efficacy of FP inhalation solution administered via nebulizer, compared with BUD suspension for inhalation, in Chinese patients with severe, persistent asthma.
Methods
Study design
This multicenter, randomized, single-blind, active-controlled, parallel-group study involved a 1:1 randomization to a 12-week treatment course of FP nebules 1 mg via nebulizer twice daily or BUD 2 mg via nebulizer twice daily.
The study was performed at 26 centers in China over a total of 14 months, from September 27, 2012, and completed on November 7, 2013. The study protocol, amendments, and informed consent were reviewed and approved by an investigational center ethics committee. This study was conducted in accordance with the International Conference on Harmonisation Guidelines and all applicable subject privacy requirements and ethical principles outlined in the Declaration of Helsinki [2008]. Written informed consent was obtained from each subject prior to the performance of any study-specific procedures.
Patients
Eligible study participants were Chinese outpatients ≥17–70 years of age, with a documented clinical history of asthma for ≥12 weeks prior to the first visit based on the Guidelines of Asthma Management and Prevention 2008 (China). Patients must have demonstrated ≥12% and ≥200 mL reversibility of forced expiratory volume in one second (FEV1) within 15–30 min following inhalation of 200–400 µg of salbutamol aerosol within 12 min, prior to Visit 1 or at the screening visit, as well as meeting the following eligibility criteria: pre-bronchodilator FEV1% predicted between ≥40% and <80% at Visit 1, receiving a stable high-dose ICS for ≥2 weeks or moderate-dose ICS plus long-acting beta agonist (LABA), asthma control test score <20 at Visit 1, and provision of informed consent.
Major exclusion criteria were: a history of life-threatening asthma; bacterial or viral infection of the upper or lower respiratory tract, sinus, or middle ear not resolved within 4 weeks of the first visit and that led to a change in asthma management or was expected to affect the subject’s asthma status or ability to participate in the study; current evidence of pneumonia, pneumothorax, atelectasis, pulmonary fibrotic disease, bronchopulmonary dysplasia, chronic bronchitis, emphysema, chronic obstructive pulmonary disease, or other respiratory abnormalities other than asthma; or any clinically significant, uncontrolled condition or disease thought to put the patient’s safety at risk through study participation or that would confound the study results if the condition/disease exacerbated during the study. Patients were not eligible for enrollment if they were pregnant or lactating, if there was visual evidence of candidiasis at Visit 1 or any evidence of alcohol abuse, if they were a current smoker, or had a smoking history of ≥10 pack years – subjects should not have used inhaled tobacco products with 3 months prior to enrollment. Other exclusion criteria included: known or suspected hypersensitivity to corticosteroids or study drug excipients; any pre-planned surgical operation within 6 months; AST/ALT ≥2× ULN or ALP/bilirubin >1.5× ULN; QTc ≥450 or ≥480 msec for patients with bundle branch block at screening, and use of restricted agents or any other investigational treatment. The immediate family members of a participating investigator, study coordinator, or employee of a participating investigator were excluded from the study and patients were not permitted to perform night shift work from Visit 1 until completion.
Interventions
The study included a run-in period (2 weeks), a treatment period (12 weeks), and a follow-up period (2 weeks). During the 2-week run-in period, subjects continued ICS or ICS/LABA combination maintenance treatment at the current dose and received salbutamol aerosol inhaler for symptom rescue; additionally, subjects recorded morning and evening PEF, daytime and nighttime symptom scores (Table S1) and use of rescue medication. All medical conditions and relevant medications were recorded by investigators. Subjects were allowed to continue ICS or ICS/LABA treatment.
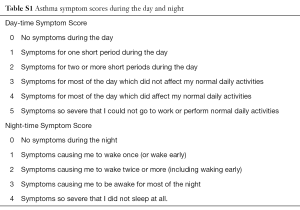
Full table
At the end of the run-in period (Visit 2), patients were eligible to enter the study if they had reported in a diary card their symptoms of asthma and/or daily salbutamol use on ≥4 of the last 7 consecutive days; showed compliance with completion of diary card reporting; and had an ACT score <20 and pre-bronchodilator FEV1% predicted <80%. At Visit 2, subjects who met the randomization criteria were randomly allocated in a 1:1 ratio to receive either FP nebules 1 mg via nebulizer twice daily or BUD respules 2 mg via nebulizer twice daily for a treatment period of 12 weeks. Both treatment interventions were administered as oral inhalation via nebulizer using the PARI BOY SX and LC SPRINT (PARI GmbH and PARItec GmbH, Germany) administration devices. Two weeks after completion of study treatment or early withdrawal, a telephone follow-up was performed to assess post-treatment AEs.
Randomized subjects were trained by unblinded staff to ensure correct use of the nebulizer and instructed to receive treatment at the same time each day during the treatment period. Subjects recorded morning and evening PEF, daytime and nighttime symptom scores, and use of rescue medication on diary cards. Twenty-four-hour urine collection for analysis of cortisol in a central laboratory was performed in a subset of subjects at selected sites. Collection was attempted within 2 days prior to each of the following visits: Visit 2 (randomization) and Visit 6 (treatment completion). All medical conditions and relevant medications were recorded in medical records. Investigators assessed lung function and reviewed diary cards at clinic visits at weeks 2, 4, 8, and 12 of the treatment period. If subjects met the criteria for pre-defined asthma control at weeks 4 or 8, they were allowed to receive half of the dose of study medication. During the study, patients were allowed salbutamol sulfate aerosol inhaler rescue medication as needed.
Subjects were withdrawn due to lack of efficacy if they experienced a severe exacerbation, at the investigator’s discretion, or if they met any of the following criteria: clinic FEV1 below FEV1 stability limit value calculated at Visit 2; PEF below PEF stability limit calculated at Visit 2 on ≥4 of 7 days or ≥8 occasions/days of salbutamol use during the 7 days immediately preceding any study contact; severe exacerbation, defined as deterioration of asthma requiring the use of systemic corticosteroids for ≥3 days, in-patient hospitalization or emergency department visit due to asthma that required systemic corticosteroids.
A protocol amendment was applied to all study centers on August 10, 2012 which included assessment of steady-state plasma pharmacokinetics of FP inhalation solution following 1 mg BID administration. Blood samples were taken at Visit 3 for this purpose. Additionally, in May 2013, one study center, the Third Affiliated Hospital of the Third Military Medical University, was added to the study after finalization of the protocol.
Outcomes
The primary endpoint was mean change in morning PEF from baseline over the 12-week treatment period. Secondary endpoints included FEV1, mean change from baseline over the 12-week treatment period in evening PEF, and percentage of symptom-free 24-hour periods and rescue-free 24-hour periods from baseline over the 12-week treatment period. FEV1 was measured electronically by spirometry at Visits 1–6, and the highest of three technically acceptable measurements was recorded. FEV1 was measured prior to study medication administration and any rescue medication use. Other secondary endpoints were median daytime and nighttime symptom scores and median number of uses of rescue medication over the 12-week treatment period. Safety assessments comprised reporting of AEs, clinical laboratory tests, oropharyngeal examinations, 24-hour urinary cortisol, vital signs, electrocardiograms, and physical examinations. AEs were coded using MedDRA and classified as pre-treatment (AE start date ≤ first dose date –1), on-treatment (first dose date ≤ AE start date ≤ last dose date +1) and post-treatment (AE start date ≥ last dose date +2).
Sample size
A total set of 300 patients was required to be randomized to achieve 240 evaluable subjects or 120 evaluable subjects per treatment group (PP population). This sample size had 80% power to reject the null hypothesis that FP inhalation solution 1 mg BID was inferior to BUD suspension for inhalation 2 mg BID with regard to the primary efficacy endpoint using a one-sided t-test at significance level 2.5% and assuming that the true treatment difference (FP minus BUD) was 3 L/min, the non-inferiority margin was –12 L/min and the common standard deviation was 40 L/min. It was estimated that 24 subjects would be required to undergo pharmacokinetic sampling in order to obtain 12 subjects who received FP inhalation solution treatment and provided valid blood samples. The software used for sample size calculations was NCSS Trial, PASS 2005, and GESS Trial.
Randomization and blinding
Each subject was assigned a unique subject number at Visit 1. At Visit 2, eligible subjects were randomized to a treatment group through a telephone call to the Registration And Medication Ordering System which provided each subject with their randomization number (from a randomization schedule generated by GlaxoSmithKline) and treatment pack number that identified the single-blind medication.
The allocation of study medication was single-blind to investigators. Independent un-blinding site staff handled study drug-related actions, and independent un-blinding staff in the central laboratory were assigned to observe PK sample number.
Statistical analysis
The intent-to-treat (ITT) population comprised all subjects randomized to treatment who received at least one dose of study medication. The per protocol (PP) population included all subjects in the ITT population who did not have any full protocol violations that could impact treatment effect. Protocol deviations were either full or partial; subjects with only partial deviations were considered part of the PP population, but from the date of their deviation their data were excluded from the PP analyses. The urinary cortisol (UC) population comprised a subset of subjects from the ITT population who did not have protocol violations considered to influence the UC endpoint and whose urine samples were not considered to have confounding factors that would affect interpretation of results.
Efficacy analyses were performed on both the ITT and PP populations. For the primary efficacy endpoint, results from both the ITT and PP populations are reported. For other efficacy endpoints, only ITT population results are reported. Mean change in morning PEF from baseline over the 12-week treatment period was analyzed using an analysis of covariance (ANCOVA) model with effects due to baseline morning PEF, center, gender, age and treatment. The least squares (LS) means for each treatment and estimated treatment difference for the treatment comparisons are presented with 95% CI for the difference and P values. Mean change in evening PEF, percentage symptom-free 24-hour periods, and percentage rescue-free 24-hour periods were analyzed using a similar ANCOVA model. Median daytime and nighttime symptom scores and median rescue medication use were analyzed using the Wilcoxon-rank sum test. A repeated measures model including baseline, gender, center, age, visit, treatment, visit-by-treatment, and visit-by-baseline was used for analysis of change from baseline in FEV1. Percentage change in mean morning week 12 PEF and FEV1 from baseline was calculated as part of the post hoc analysis.
Primary endpoint analyses included only subjects with ≥4 days of non-missing data in the baseline week prior to randomization and ≥4 days of non-missing data after randomization. Weekly means were considered missing if <2 days were recorded in a given week. Statistical analysis and generation of tables, figures, and listings were performed using the SAS software package version 9.1.3 or later.
Results
Patients
A total of 460 patients were screened for eligibility and 351 included in the run-in period. Of those, 317 were randomized: 159 to the FP treatment group and 158 to the BUD treatment group (Figure 1). One subject from each treatment group did not receive the allocated intervention. Of subjects in the ITT population, 80% [253] completed the study and the early withdrawal rate was comparable for both treatment groups (FP: 22%; BUD: 17%). The most common primary reason for withdrawal after randomization was reaching protocol-defined stopping criteria (FP: 5%; BUD: 6%). A total of 7% and 3% of subjects in the FP and BUD groups, respectively, withdrew after randomization due to adverse events.
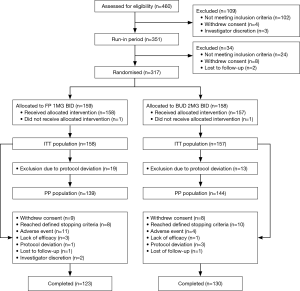
Full protocol deviations occurred in 12% and 8% of subjects in the FP and BUD groups, respectively, and these patients were excluded from the PP population. Full protocol deviations included poor overall compliance, prohibited medication prior to or at randomization or violation of exclusion/inclusion criteria. Partial protocol deviations occurred in 11% and 17% of subjects in the FP and BUD groups, respectively. Partial protocol deviations included PEF below limit, requirement for dose reduction at Visit 4 or 5 not fulfilled, dose reduction without meeting dose reduction criteria, ≥8 uses of salbutamol per day and prohibited medication use after randomization.
Baseline data
Subject demographics in the ITT population were comparable between treatment arms (Table 1): mean age was similar between treatment groups (FP: 51.7 years; BUD: 51.1 years); all subjects were Chinese, and males comprised 51% of the FP group and 55% of the BUD group. Asthma history was also similar between treatment groups, with a mean duration of 11.26 and 11.50 years for the FP and BUD groups, respectively. Screening lung function tests showed that the mean actual measurement of FEV1 was 1.510 L (FP: 1.507 L; BUD: 1.514 L) and mean percent predicted FEV1 (American Thoracic Society standard with a correction factor for Chinese race of 0.88) (10) was 61.87% (FP: 62.02%; BUD: 61.72%). At randomization, the mean actual measurement of FEV1 was 1.529 L (FP: 1.520 L; BUD: 1.538 L) and mean percent predicted FEV1 was 62.67% (FP: 62.51%; BUD: 62.83%).
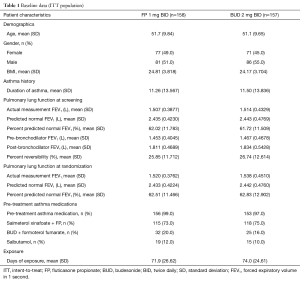
Full table
The majority of subjects took asthma medication pre-treatment [salmeterol xinafoate + FP (FP: 73%; BUD: 75%), BUD + formoterol fumarate (FP: 20%; BUD: 16%), salbutamol (FP: 12%; BUD: 10%)]. Few subjects took concomitant asthma medications during treatment (FP: 4% and BUD: 5%). The mean (and median) days of treatment exposure was similar between the two treatment groups [FP: 71.9 (84.0) and BUD: 74.0 (84.0)] as was the mean treatment compliance rate (FP: 96.6% and BUD: 96.9%). The majority of subjects (FP: 72% and BUD: 74%) were 95–105% compliant, with few subjects <80% compliant (FP: 7% and BUD: 6%).
Efficacy
At baseline, mean morning PEF in the ITT population was 245.7 and 257.0 L/min in the FP and BUD treatment groups, respectively. At week 12, the mean morning PEF increased by 26.7 L/min (14.1%) and 28.0 L/min (15.3%), respectively (Figure 2A). The LS mean change in morning PEF for the ITT population was 12.71 L/min for the FP group and 14.51 L/min for the BUD group. The LS mean change difference (FP minus BUD) was –1.80 L/min (95% CI: –12.19, 8.59; P=0.733). The lower limit of the 95% CI was –12.19 L/min, which was not above the pre-specified non-inferiority margin of –12 L/min, but the difference of –0.19 was small and not clinically meaningful.

Mean morning PEF in the PP population at baseline was 245.6 L/min in the FP group and 260.8 L/min in the BUD group. At week 12, this increased by 29.1 L/min (15.7%) and 30.1 L/min (16.2%) in the FP and BUD groups, respectively (Figure 2B). The LS mean change in morning PEF from baseline over the 12-week treatment period was 13.50 L/min for the FP group and 15.78 L/min for the BUD group. The LS mean change difference (FP minus BUD) was –2.28 L/min (95% CI: –12.95, 8.38; P=0.674), with the lower limit of the two-sided 95% CI being –12.95 L/min, which was not above the pre-specified non-inferiority margin of –12 L/min, but the difference of –0.95 L/min was small and not clinically meaningful. For the primary efficacy endpoint, interactions with treatment were explored for baseline, gender, age, and center in the ITT and PP populations and no statistically significant interactions found.
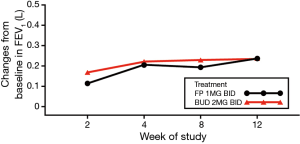
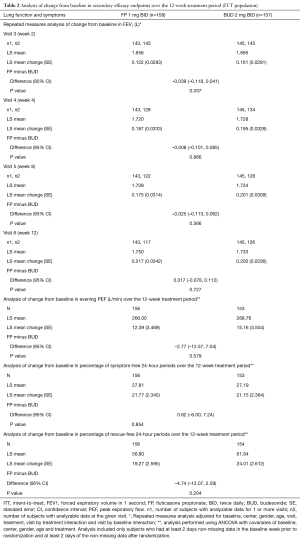
Baseline FEV1 was similar between treatment groups, and mean FEV1 increased over time in a similar manner in both treatment groups. At week 12, mean FEV1 increased by 0.237 L (16.79%) and 0.236 L (17.73%) in the FP and BUD groups, respectively (Figure 3; Table 2). The P value for the treatment comparison was > 0.3 at each visit, indicating a non-statistically significant difference between treatment groups at all visits.
Full table
Baseline evening PEF was 248.6 and 257.1 L/min in the FP and BUD groups, respectively. Over weeks 1–12, the evening PEF increased similarly in the two treatment groups. The LS mean change in evening PEF from baseline over the 12-week treatment period was 12.39 L/min for the FP group and 15.16 L/min for the BUD group [difference (FP minus BUD) =–2.77; P=0.579].
At baseline, the mean percentage of symptom-free 24-hour periods was 5.1% and 7.2% in the FP and BUD groups, respectively. The LS mean change in percentage of symptom-free 24-hour periods from baseline over the 12-week treatment period was 21.77% for the FP group and 21.15% for the BUD group and the difference in LS mean change (FP minus BUD) was 0.62% (P=0.854). The median (and mean) daytime symptom scores over the 12-week treatment period were 1.0 (1.1) and 1.0 (1.0) in the FP and BUD groups, respectively, with no statistically significant difference between groups (P=0.123). The median (and mean) nighttime symptom scores over the 12-week treatment period were 1.0 (0.8) and 1.0 (0.8) for the FP and BUD treatment groups, respectively, also without a statistically significant difference between groups (P=0.949). At baseline, the mean percentage of rescue-free 24-hour periods was 40.3% and 35.2% in the FP and BUD groups, respectively. The LS mean change was 19.27% for the FP group and 24.01% for the BUD group and LS mean change difference (FP minus BUD) was –4.74% (P=0.204). The median (and mean) values for rescue medication usage were 0.0 (1.0) and 0.0 (0.7) for the FP and BUD groups, respectively (P=0.170).
Post hoc analyses
Post hoc analyses of within-group comparison of change from baseline in morning PEF were performed. Marked improvements in morning PEF were found over weeks 1–4, 1–8, and 1–12 for both treatment groups in the ITT population. In the FP group, the mean changes from baseline in morning PEF for weeks 1–4, 1–8, and 1–12 were 6.41, 10.50, and 12.98 L/min, respectively. In the BUD group, the mean change from baseline in morning PEF for weeks 1–4, 1–8, and 1–12 was 6.86, 11.91, and 14.22 L/min, respectively. All changes in both treatment groups were statistically significant (P<0.05). Treatment comparison of change from baseline in morning PEF over weeks 1–4 showed that the lower limits of the 95% CI for the LS mean treatment difference over weeks 1–4 were –10.41 and –11.96 L/min in the ITT and PP populations (Table 3). Both limits were above –12 L/min.

Full table
For the analysis of change from baseline in morning PEF over the 12-week treatment period, asthma duration, in addition to baseline morning PEF, center, gender, age, and treatment, was also included in the ANCOVA model. The lower limit of the two-sided 95% CI for the LS mean treatment difference (FP minus BUD) of adjusted change from baseline in morning PEF over the 12-week treatment period was –11.22 and –11.92 L/min, in the ITT and PP populations respectively, which were both above the pre-specified non-inferiority margin of –12 L/min.
Safety
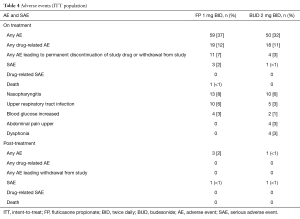
No trends were noted in review on the on-treatment AEs. On-treatment AEs were reported for 59 subjects (37%) in the FP group and 50 subjects (32%) in the BUD group (Table 4). AEs considered by the investigator to be related to the study drug were reported in 19 subjects (12%) in the FP group and 18 subjects (11%) in the BUD group. All on-treatment AEs were of mild or moderate intensity except three events of severe intensity (nasopharyngitis, asthma exacerbation, and nephrotic syndrome) and one event (angina pectoris) reported as intensity not applicable. On-treatment SAEs occurred in three subjects (2%) in the FP group and one subject (<1%) in the BUD group. The SAEs were asthma exacerbation [FP: 1 (<1%), BUD: 1 (<1%)]; infection [BUD: 1 (<1%)], spondylolisthesis [FP: 1 (<1%)] and nephrotic syndrome [FP: 1 (<1%)]. No SAEs were considered related to the study drug. One subject in the FP group who discontinued the treatment for an unknown reason without informing the investigator suffered wheeze, cyanosis, profuse sweating, and unconsciousness, and showed no response to inhaled salbutamol, then died of asthma exacerbation, which was considered by the investigator unrelated to study drug.
Full table
No trends were noted in review of the post-treatment AEs. Post-treatment AEs were reported in three subjects (2%) in the FP group and one subject (<1%) in the BUD group. The most frequently reported was asthma exacerbation [one subject (<1%) in each treatment group] and none was considered related to the study drug. Post-treatment SAEs occurred in one subject (<1%) in each treatment group. In the FP group, one subject experienced infection and asthma, and in the BUD group one subject experienced lung infection and asthma exacerbation. None of the post-treatment SAEs was deemed related to study drug.
Eleven subjects (7%) in the FP group and four subjects (3%) in the BUD group permanently discontinued study drug or withdrew from the study due to AEs. The most frequently reported of these were upper abdominal pain [BUD: 2 (1%)], dry mouth [FP: 1 (<1%), BUD: 1 (<1%)] and cough [FP: 1 (<1%), BUD: 1 (<1%)]. There were no evident changes from baseline in chemistry and hematology values and no relevant treatment differences were observed.
A total of 111 subjects (FP: 54; BUD: 57) were included in the UC population for analysis of 24-hour UC. The LS geometric mean ratios were 0.93 and 0.48 for the FP and BUD groups, respectively. The treatment ratio was 1.93 (95% CI: 1.34, 2.79; P<0.001). The percentage of subjects with a UC concentration below normal range at week 12 was lower in the FP group (6%) than the BUD group (21%).
Thirteen subjects in the FP group were included in the pharmacokinetic population, used for the pharmacokinetic analysis of FP. Following administration of FP, the maximum plasma concentration was achieved at a median Tmax of 0.83 h post-dose. The geometric means of the AUC (0–tau) and Cmax were 403.10 pg.h/mL and 59.24 pg/mL, respectively, and the inter-subject coefficients of variation for AUC (0–tau) and Cmax were 70.5% and 115.0%, respectively.
Discussion
In this study, the mean morning PEF increased over time, with clinically significant improvements in both treatment groups. At week 12, the mean morning PEF increased by 26.7 L/min (14.1%) and 28 L/min (15.3%) in the ITT population and by 29.1 L/min (15.7%) and 30.1 L/min (16.2%) in the PP population, in the FP and BUD treatment groups, respectively. The increase in morning PEF was above the patient-perceivable improvement and clinically significant value of 18.8 L/min (10). The lower limit of the 95% CI for the LS mean treatment difference was –12.19 L/min in the ITT population and –12.95 L/min in the PP population. These values overstepped the pre-specified non-inferiority margin of –12 L/min by only 0.19 and 0.95 L/min. Although non-inferiority was not demonstrated according to the pre-specified criteria, the extent that the values exceed the margin was small and not clinically meaningful. The absolute values for the lower limit of difference were less than the patient perceivable improvement and clinically significant value, suggesting that the difference between treatment groups was not clinically significant.
Asthma duration may be related to the historical exposure of ICS and resulting airway remodeling, which may influence response to treatment. Post hoc analyses that adjusted for the effects of asthma duration revealed that the lower limit of the two-sided 95% CI for change from baseline in morning PEF over the 12-week treatment period was within the pre-specified non-inferiority margin of –12 L/min.
The results above indicate that both FP inhalation solution and BUD suspension for inhalation improve morning PEF for patients with severe, persistent asthma. In addition, FEV1 and PEF have a close relationship; both used in the routine diagnosis and clinical assessment of asthma severity (1). In this study, the mean FEV1 increased over time similarly in both treatment groups: at week 12, the mean FEV1 increased by 0.237 L (16.8%) and 0.236 L (17.7%) in the FP and BUD groups, respectively, and the morning PEF and FEV1 results demonstrate that both FP inhalation solution and BUD suspension for inhalation improved lung function in this patient group.
The assumed treatment difference used for sample size calculation was based on historical study results from other inhaled formulations of FP and BUD. A 1998 meta-analysis including seven randomized controlled trials comparing FP with BUD showed that FP was associated with greater improvement in morning PEF compared with BUD at clinically equivalent doses, with an overall difference of +11 L/min (95% CI: 7, 15) (6). A 2007 systematic review of FP compared with BUD or BDP showed similar results (4).
Possible reasons for the smaller primary endpoint treatment difference in morning PEF improvement and wider confidence intervals observed in this study may be that this was the first study to compare nebulized FP and BUD in patients with severe, persistent asthma and all statistical hypotheses were based on historical study data using other formulations of FP and BUD. Additionally, the individual variance of response to steroid therapy from different patients with severe, persistent asthma is large, which may lead to increased standard deviation in study results. These reasons may have caused the smaller than expected treatment difference (FP minus BUD) in morning PEF improvement, together with a residual standard deviation higher than the planned value which contributed to a wider confidence interval, resulting in the failure to demonstrate non-inferiority.
The safety data indicated that the rates of AEs, SAEs and treatment-related AEs were similar between treatment groups. The incidence and type of AEs were in line with those reported in previous studies (11). The vast majority of AEs were considered unrelated to the study drug and most were of mild or moderate intensity. FP inhalation solution had a minor effect on UC levels and no new safety issues were identified; UC decreased by 7% from baseline in the FP group and 52% from baseline in the BUD group (P<0.05). A previous study showed no difference in UC levels with treatment with inhaled formulations of FP and BUD for 12 months, although the doses and dose ratio differed (12).
The pharmacokinetic analyses of FP demonstrated low systemic exposure following 1 mg BID administration. This finding is supported by previous studies demonstrating a low level of oral bioavailability, which reduces the potential for FP to cause systemic effects (13).
Conclusions
The results of this study established the efficacy and safety of FP inhalation solution administered via nebulizer for the treatment of severe, persistent asthma. Although the mean change in morning PEF from baseline did not meet the pre-specified criteria for non-inferiority, the point estimate difference was small and not clinically meaningful. Twelve weeks’ treatment with FP resulted in clinically meaningful improvements in morning PEF and FEV1, indicating improved lung function in Chinese patients with severe, persistent asthma. These results confirm the findings of other studies that have found FP inhalation solution to be an efficacious treatment for moderate-to-severe asthma.
Acknowledgements
Funding for this study was provided by GlaxoSmithKline (China) R&D Co., Ltd. All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. We would like to thank Xiangdong Zhou, Limin Wang, Shuang Liu, Yuejian Liu, Xiaoyun Hu, Lin Ma, Ziwen Zhao, Fuxin Hui, Xiwei Zheng, Yanping Yang, Shuyang Zhu, Ping Chen, Huanying Wan, Changhui Wang, Bin Wu, Suiyang Zhang, Yong He, Yiqiang Peng, and Kejing Ying for their contributions to the study design and conduct. We are also grateful for the devotion of all study staff to the conduct of this study. Development of the initial outline and draft, collation of authors’ comments, assembly of figures and tables, referencing, copy editing, and graphic services were provided by MediTech Media Asia Pacific Pte Ltd and funded by GlaxoSmithKline (China) R&D Co., Ltd. ClinicalTrials.gov: NCT01687283.
Footnote
Conflicts of Interest: Sponsorship and provision of all investigational products for use in this study was provided by GlaxoSmithKline (China) R&D Co., Ltd. JL has received speaker’s and consultancy fees and has had study involvement with AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. PC has received speaker’s fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. ChL has received speaker’s and consultancy fees and has had study involvement with AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, MSD and Novartis. JK has received fees for study involvement from Boehringer Ingelheim and speaker’s and consultancy fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. WX has received speaker’s fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. ZC has received speaker’s and/or consultancy fees from AstraZeneca, Bayer, Eli Lilly, and GlaxoSmithKline. HT has received speaker’s fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. XD, CL and LL are employees of GlaxoSmithKline (China) R&D Co., Ltd.
References
- Global Strategy for Asthma Management and Prevention. Avialiable online: http://www.ginasthma.org/
- To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012;12:204. [Crossref] [PubMed]
- Su N, Lin J, Liu G, et al. Epidemiological investigation about the control level of patients with bronchial asthma in 8 provinces of China. National Medical Journal of China, 2014;53:601-6.
- Adams N, Lasserson TJ, Cates CJ, et al. Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children. Cochrane Database Syst Rev 2007.CD002310. [PubMed]
- Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000;343:332-6. [Crossref] [PubMed]
- Barnes NC, Hallett C, Harris TA. Clinical experience with fluticasone propionate in asthma: a meta-analysis of efficacy and systemic activity compared with budesonide and beclomethasone dipropionate at half the microgram dose or less. Respir Med 1998;92:95-104. [Crossref] [PubMed]
- Kolasani BP, Lanke VM, Diyya S. Influence of delivery devices on efficacy of inhaled fluticasone propionate: a comparative study in stable asthma patients. J Clin Diagn Res 2013;7:1908-12. [PubMed]
- Bauer A, McGlynn P, Bovet LL, et al. The influence of breathing pattern during nebulization on the delivery of arformoterol using a breath simulator. Respir Care 2009;54:1488-92. [PubMed]
- Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest 2010;137:138-45. [Crossref] [PubMed]
- Santanello NC, Zhang J, Seidenberg B, et al. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J 1999;14:23-7. [Crossref] [PubMed]
- Terzano C, Ricci A, Burinschi V, et al. Comparison of the efficacy of beclometasone dipropionate and fluticasone propionate suspensions for nebulization in adult patients with persistent asthma. Respir Med 2003;97 Suppl B:S35-40.
- Hughes JA, Conry BG, Male SM, et al. One year prospective open study of the effect of high dose inhaled steroids, fluticasone propionate, and budesonide on bone markers and bone mineral density. Thorax 1999;54:223-9. [Crossref] [PubMed]
- Harding SM. The human pharmacology of fluticasone propionate. Respir Med 1990;84 Suppl A:25-9.


