Avoiding completion pneumonectomy by omentopexy for bronchial dehiscence
Introduction
Bronchoplasty has become the standard surgical procedure for treatment of non-small cell lung cancer (NSCLC) (1). Induction chemoradiotherapy (CRT) is also sometimes performed for locally advanced NSCLC. Several papers have reported that the incidence of perioperative anastomotic complications after sleeve lobectomy was not increased after neoadjuvant CRT, even in unprotected situations (2-4). However, anastomotic complications such as bronchial dehiscence remain problematic, especially after CRT. Completion pneumonectomy (CP) is sometimes needed in these situations but is associated with high morbidity and mortality (5). We herein report our experience with omentopexy for bronchial dehiscence after sleeve lobectomy. This procedure helped to avoid CP.
Case presentation
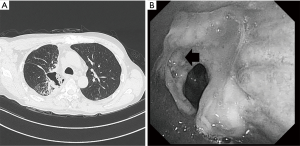
A 56-year-old man with a cough and fever exhibited a mass shadow in the right upper lung field on a chest X-ray. Transbronchial lung biopsy showed squamous cell carcinoma. Systemic evaluation revealed paratracheal lymph node (#4R) swelling and FDG accumulation, leading to a diagnosis of clinical T2aN2M0 stage IIIA cancer. After concurrent induction CRT [two cycles of S-1 (80 mg/m2) + cisplatin (60 mg/m2), 40 Gray] and achievement of a partial response according to the Response Evaluation Criteria in Solid Tumors, the patient was referred to our hospital for surgical treatment. A right posterolateral thoracotomy was performed. The pulmonary arteries and veins were divided. The tumor had invaded the orifice of the upper lobe bronchus, then the two cartilage rings were resected both proximal and distal side. After we divided pulmonary ligament, an interrupted bronchial anastomosis was performed with 4-0 monofilament absorbable sutures (PDS-II; Ethicon, Inc., Somerville, NJ, USA) with a pedicle-pericardial fat pad after systemic mediastinal lymph node dissection. There was no tension in the anastomosis. He suddenly developed high grade fever, and cough. Chest CT revealed that the patient developed anastomotic dehiscence 11 days postoperatively (Figure 1A). Bronchoscopy showed leakage from the anastomosis (Figure 1B). Initially, conservative treatment including intravenous prostaglandin E1 was attempted because antibiotic therapy was effective without chest tube drainage and the patient was in a good general condition due to the localized empyema space. Once, he could discharge on 6 weeks postoperatively.
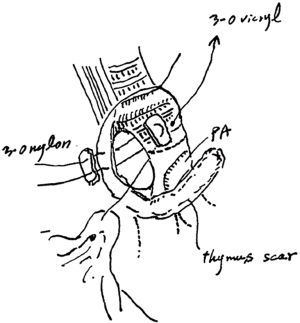
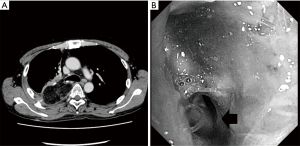
He developed a fever and cough 8 weeks postoperatively, and a redo operation was performed. After upper laparotomy, an omental flap was harvested with a good blood supply from the right gastroepiploic vessels and it was guided to pleural space through the sub-xiphoid space. A right posterolateral thoracotomy was then performed along the previous scar. A fistula measuring 10 mm in diameter was found around the transition region between the cartilage and membranous portions of the bronchus after dissection of severe adhesion in the pleural space. As the fistula was not sutured directly, a single 3-0 nylon suture with a pledget was placed to facilitate closure of the bronchial wall, and the tip of the omentum was inserted into the fistula and fixed around the surface of the residual lung (Figure 2). The patient’s condition improved after this redo operation with surveillance bronchoscopy to check the anastomotic status. Several intervention were required to get rid of the granuloma around the anastomosis, it has been remained to be a good passage. Chest computed tomography showed no residual air space and a well-vascularized omentum in the chest cavity (Figure 3A). Bronchoscopy showed no air leakage 8 weeks after the redo operation (Figure 3B).
Discussion
This report describes the successful treatment of bronchial dehiscence after induction CRT with subsequent sleeve lobectomy by omentopexy, resulting in preservation of lung function.
Surgical resection is sometimes performed even for locally advanced NSCLC after induction chemotherapy, radiotherapy, or both. Bronchial sleeve lobectomy has been attempted in such cases and accepted as an effective alternative to pneumonectomy and had good oncological outcomes (1). However, the induction therapy is detrimental to wound healing, especially bronchoplasty. The reported incidence of bronchial complications is approximately 0.0% to 10.8% (1-4). This situation sometimes requires CP, which is associated with high morbidity and mortality; once successfully treated, the patient’s quality of life may still significantly decrease. In one study, the incidence of bronchopleural fistula was 11.0% and the perioperative mortality rate was 5.7% after CP (5). On the other hand, several high-volume institutional studies have found that the incidence of perioperative anastomotic complications after sleeve lobectomy does not increase after neoadjuvant CRT (2,4). In their meta-analysis, however, Li et al. (6) showed that CRT was significantly associated with the incidence of bronchopleural fistula (odds ratio of 2.53); thus, most surgeons recognize the risk of anastomotic complications after induction CRT. Several techniques with which to protect the anastomosis have been described, including application of patches using the pericardial fat pad, thymus, intercostal muscle, and greater omentum (7). However, these prophylactic methods still remains controversial (3). Meyer et al. (8) have reported the excellent results by using latissimus dorsi or serratus anterior muscles flaps for repair of airway defect. But we could not use it for redo-thoracotomy because we usually cut latissimus dorsi by posterolateral thoracotomy. On the other hand, the omentum has been the most widely used and is the most effective tissue for this purpose because it is rich in fat and provides sufficient blood flow to the mucosal tissue (7). In fact, we prefer using omentum in the empyema like this case, because large amount of fat tissue could fil in the empyema space and also facilitated wound healing at bronchial anastomosis (9). Clinical reports also have described using the greater omentum for anastomotic dehiscence of the airway (10).
We initially thought that CP was inevitable in the present case. In fact, the patient had sufficient pulmonary function before the initial operation (forced expiratory volume in 1 s =3,120 mL), and after the operation, he had a good performance status and no comorbidity. We therefore thought that he was suitable for CP. However, the patient insisted that the “healthy” right middle and lower lobes should be preserved to maintain his quality of life after discharge because pathological examination of the specimen showed no residual tumor, and no adjuvant therapy was required. Thus, we performed an omentopexy to preserve the patient’s residual lung function and maintain his quality of life. His condition improved after the redo operation. We believe that this outcome was facilitated by the following. First, the patient was relatively young, had no comorbidities, and had a good performance status even after the initial operation. Second, he had no history of laparotomy, and abdominal CT predicted that he had a fat-rich greater omentum (body mass index of 22.3 kg/m2). Third, the small empyema space located between the residual middle and lower lobe expanded well in the chest cavity, improving his condition.
In conclusion, omentopexy may be an effective treatment option that may help to avoid CP in selected patients. However, this procedure requires subsequent surveillance bronchoscopy to check the anastomotic status.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Nagayasu T, Yamasaki N, Tsuchiya T, et al. The evolution of bronchoplasty and broncho-angioplasty as treatments for lung cancer: evaluation of 30 years of data from a single institution. Eur J Cardiothorac Surg 2016;49:300-6. [Crossref] [PubMed]
- Gonzalez M, Litzistorf Y, Krueger T, et al. Impact of induction therapy on airway complications after sleeve lobectomy for lung cancer. Ann Thorac Surg 2013;96:247-52. [Crossref] [PubMed]
- Storelli E, Tutic M, Kestenholz P, et al. Sleeve resections with unprotected bronchial anastomoses are safe even after neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:77-81. [Crossref] [PubMed]
- Milman S, Kin AW, Warren WH, et al. The incidence of perioperative anastomotic complications after sleeve lobectomy is not increased after neoadjuvant chemoradiotherapy. Ann Thorac Surg 2009;88:945-50; discussion 950-1. [Crossref]
- Puri V, Tran A, Bell JM, et al. Completion pneumonectomy: outcomes for benign and malignant indications. Ann Thorac Surg 2013;95:1885-90; discussion 1890-1.
- Li S, Fan J, Liu J, et al. Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: a systematic meta-analysis of 14912 patients. Jpn J Clin Oncol 2016;46:534-46. [Crossref] [PubMed]
- Masaoka T, Oizumi H, Fujishima T, et al. Removal of cartilage rings prevents graft stenosis in extended tracheal allotransplantation with omentopexy and immunosuppression: an experimental study. J Heart Lung Transplant 2002;21:485-92. [Crossref] [PubMed]
- Meyer AJ, Krueger T, Lepori D, et al. Closure of large intrathoracic airway defects using extrathoracic muscle flap. Ann Thorac Surg 2004;77:397-404; discussion 405. [Crossref] [PubMed]
- Tomita M, Ayabe H, Kawahara K, et al. Benefit from omentopexy on bronchial wound healing in performing concurrent esohagectomy. Acta Med Nagasaki 1988;33:87-90.
- Okada M, Kawaraya N, Kujime K, et al. Ometopexy for anastomotic dehiscence after tracheal sleeve pneumonectomy. Thorac Cardiovasc Surg 1997;45:144-5. [Crossref] [PubMed]


