Impact of cyclooxygenase-2 and prostaglandin-E2 expression on clinical outcome after pulmonary metastasectomy
Introduction
Nearly 1.4 million people are annually diagnosed with colorectal cancer (CRC), making it the third most common type of cancer worldwide (1). Metastases are considered the major contributor of colorectal cancer-related morbidity and mortality. Although the liver is the primary site of metastasis, pulmonary spreading occurs in approximately 10% of patients (2,3). Despite ongoing efforts to improve patient care and recent advances in chemotherapy the overall 5-year survival rate of patients with stage IV CRC remains low at only around 10–15% (4-6). Surgical resection of oligometastatic lesions represents the only curative option for patients with lung metastases. In carefully selected patients 5-year survival rates of 40–68% are reported (7). Commonly accepted inclusion criteria for pulmonary metastasectomy (PM) are: (I) controlled primary tumor; (II) complete resection of all metastatic lesions; (III) exclusion of disseminated, extrathoracic disease; and (IV) an adequate performance status (8).
In addition to these “traditional” criteria, different biological behaviours of CRC subtypes were proposed to be important when selecting patients for PM. Therefore, various biological markers, which could distinguish between aggressive and more benign phenotypes of pulmonary spreading are currently in the spotlight of research (9,10).
In 2011 Hanahan and Weinberg updated their model of tumor pathogenesis by adding the concept of continuous inflammation as a main component of malignant transformation and tumor growth (11). This “hallmark of cancer” includes inflammatory mediators such as cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE-2). On the other hand the immune system and its soluble factors are an important part of the body’s endogenous antitumor defence. Cells of the immune system recognize tumorous tissue and consequently eliminate tumor cells.
The physiological function of COX-2 is to mediate inflammation by converting arachidonic acid to prostaglandin H2, which is subsequently transformed to PGE2. An overexpression of COX-2 has been previously reported in primary CRC and was associated with tumor progression in various studies (12-14). Nevertheless, the expression pattern of COX-2 has not yet been addressed in the setting of PM.
Prostaglandin-E2 is an essential inflammatory mediator, but also regulates other processes such as vasodilatation or muscle relaxation. PGE2 is overexpressed in various malignancies (15), whereas its prognostic value remains elusive. Furthermore, the expression of PGE2 in pulmonary metastases of CRC and its prognostic impact after PM is currently unknown.
Methods
Study population
This study was designed as a retrospective analysis and is based on a prospectively documented and actively followed patient cohort. Data on the primary tumor, number and distribution of metastases, previous treatments, etc were collected at the time of PM and documented in our institutional PM database. A total of 53 CRC patients with pulmonary metastases undergoing curative metastasectomy from April 2009 to November 2013 were included in this study. In case of patients who had undergone a PM before the inclusion period, the specimen of the first PM was also assessed. For 26 patients paraffin embedded specimens from the primary tumor were obtained and stained. Tumor staging prior to metastasectomy was performed by abdominal and thoracic computed tomography (CT) scan. In case of inconclusive CT scans, positron emission tomography (PET) was used to exclude extrathoracic spreading. All patients were accessed through a muscle-sparing anterolateral or posterior incision. Lungs were bimanually palpated for occult lesions and a lymph nodes sampling was performed. Complete resection (R0) was achieved in all patients.
Lung metastasis free survival (LMFS) was defined as the period of time between the diagnosis of the primary tumor and the diagnosis of metastatic spread to the lungs. Time to recurrence represented the period of time between PM and the first evidence of metastatic recurrence at any site. Time to pulmonary recurrence was defined as the time between PM and the first manifestation of pulmonary recurrence detected by CT scan.
Patients were postoperatively followed-up every three months during the first year and every six months during the following years. This study was approved by the ethics committee of the Medical University of Vienna (EK#: 1097/2014) and was performed according to the Declaration of Helsinki and the Good Scientific Practice guidelines of the Medical University of Vienna.
Immunohistochemistry and scoring
Formalin fixed, paraffin-embedded tissue specimens were analyzed using standard immunohistochemistry protocols. Tissue specimens were cut in 4 µm thick sections and heat mediated antigen retrieval was performed by microwave. For suppressing the endogenous peroxidase activity, the sections were incubated in 0.3% H2O2 for 30 min at room temperature. The following primary antibodies were used and incubated for 1 hour at 4 °C: anti-COX-2 Clone CX-294 (DAKO) 1:50 and PGE2 EP4 Antibody (SantaCruz, USA) 1:25. The VECTASTAIN ABC Kit Mouse IgG and the VECTASTAIN ABC Kit Rabbit IgG (Vector Laboratories, Burlingame, California) were used according to the manufacturer’s protocol. The reaction was visualized with DAB substrate (SIGMAFAST 3,3’-Diamino-benzidine tablets) and counterstained with hematoxylin. As negative controls, the primary antibody was omitted. PGE2 and COX-2 staining failed in five cases, respectively, leaving 48 cases (91%) for further analyses.
Two independent blinded observers assessed the staining intensity as previously described (16). IHC scores were calculated by multiplying the intensity (0-negative, 1-weak, 2-moderate and 3-strong) by the percentage of positively stained tumor or stromal cells (0 to 100), resulting in IHC scores ranging from 0 to 300. In case that the two ratings differed, the stained section was discussed and re-evaluated. For some analysis the continuous IHC score was transformed into a dichotomous variable by calculating the median score for metastases and primary tumors, respectively. Tumor and stroma were defined as high-expressing (COX-2high, PGE2high) when IHC scores were equal or above the median and defined as low-expressing (COX-2low, PGE2low) when scores were below the median.
Statistical analysis
All data collected and used were evaluated using SPSS 21 (SPSS Inc., Chicago, USA). Kruskal-Wallis test was used to compare medians of two groups. Kaplan Meier curves and log rank test were used for comparison of survival functions. Cox-regression was used for multivariate analyses, including factors with a P value of <0.2 in univariate tests. Pearson correlation was applied to determine the relationship between IHC scores of pulmonary metastases and matched primaries. Chi-square test was used to compare binominal variables. If the expected frequency was below 5, Fisher‘s exact test was applied. All tests were calculated in a two-sided manner. P values of <0.05 were defined as statistically significant.
Results
Patient’s characteristics
The study collective comprised 53 patients, 22 female and 31 male patients. The primary tumor site was colon in 59% and rectum in 41% of cases. Most of the patients were already in advanced tumor stages (n=44, 83% T3/T4) and more than half of the patients had histologically confirmed lymph node spreading at the time of their primary operation (60%). Eight of the 53 patients presented with concomitant distant metastases at the time of primary diagnosis (4 liver, 4 lung). 30% of the patients were curatively treated for liver metastases prior to PM. At the time of PM the median age was 65 years (range, 45–83 years). Most of our patients presented with singular pulmonary nodules (n=41; 77%). Resection was complete for all patients with negative resection margins. Only 1 patient had a positive intrathoracic lymph node metastasis according to the final histological report. Median follow up after PM was 28 months (range, 3–125 months).
COX-2 and PGE2 are highly expressed in lung metastases and corresponding primary tumors
Five patients had to be excluded from further analysis due to improper staining of COX-2 and PGE2. COX-2 was evident in 98% of pulmonary metastases (47/48). The calculated median IHC score was 125. Corresponding primaries evidenced detectable intratumoral COX-2 levels in 96.2% available specimens with a median IHC score of 180. Representative stainings of COX-2 in pulmonary metastases are shown in Figure 1, stainings of primary CRC specimens in Figure S1. The IHC scores between primary and pulmonary metastases did not correlate as evaluated by Pearson correlation (r=0.296, P=0.171).
All analyzed patients showed a positive expression of PGE2 in their pulmonary metastases (48/48) with a median IHC score of 210. PGE2 expression was also evident in all corresponding primary. PGE2 IHC score did not correlate between primary and pulmonary metastases (Pearson r correlation =−0.121, P=0.602). PGE2 stainings of pulmonary metastases and corresponding primaries are presented in Figure 1 and Figure S1.
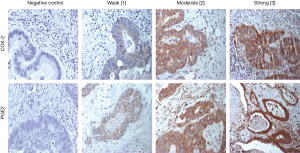
COX-2 and PGE2 are weakly expressed in peritumoral stroma of lung metastases and primary tumors
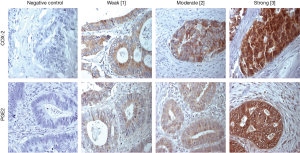
In contrast to the intralesional evaluations, stromal COX-2 expression was less common and COX-2 was only evident in 27% of pulmonary metastases (13/48). Thus, the calculated median IHC score was 0 (range, 0–90). Corresponding primaries showed a positive expression of stromal COX-2 in 28% of available specimens. Again, the IHC-levels of primary and pulmonary metastases did not correlate (Pearson r correlation =−0.06, P=0.791).
Eighty-three percent (40/48) of patients evidenced stromal expression of PGE2 in their pulmonary metastases with a median IHC score of 82.5. Stromal PGE2 expression was also evident in 83% corresponding primary, however, IHC scores between primary and pulmonary metastases did not correlate (Pearson r correlation =−0.322, P=0.143).
Representative stainings of stromal COX-2 and PGE2 expressions in pulmonary metastases are shown in Figure 2 and for primary CRC specimens in Figure S2.
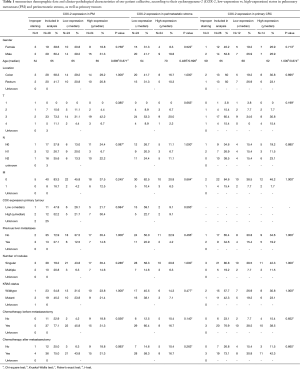
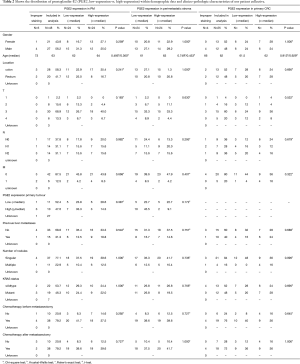
Distribution of COX-2 and PGE2 expression within clinicopathological characteristics
In order to facilitate the comparison between different COX-2 and PGE2 expression levels, patients with IHC scores equal or above the median were assigned to a high expression-group and those with IHC scores below the median were defined as low expressing. The impact of clinical characteristics on COX-2 and PGE2 expression was evaluated. No correlations between expression of these two inflammatory markers with gender, age, location of the primary tumor (colon vs. rectum), T stage, tumor grading, prior liver metastases and number of pulmonary nodules could be found (Tables 1,2). However, we found that patients who had not received chemotherapy before PM had higher IHC scores of COX-2 compared to patients with a prior chemotherapy (P=0.036).
Full table
Full table
Impact of COX-2 and PGE2 expression on outcome parameters
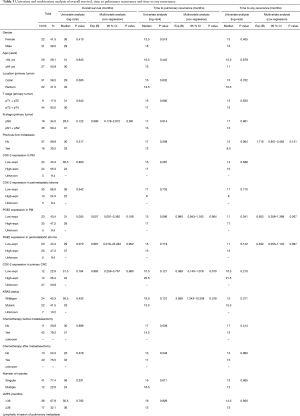
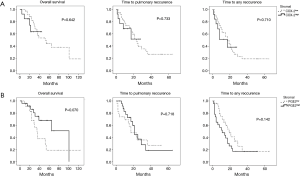
The impact of COX-2 and PGE2 expression on clinical outcome after PM was analysed next. Kaplan Meier curves showed similar survival curves for overall survival, but a trend towards prolonged time to pulmonary recurrence and time to any recurrence in the COX high-expressing group (Figure 3, Table 3). Expression level of PGE2 in pulmonary metastases tissue also had an impact on the time to pulmonary recurrence, the time to any recurrence and the overall survival. Again, Kaplan Meier analyses showed a favourable outcome of PGE2 high expressing tumors in terms of overall survival and time to recurrence (Figure 3, Table 3). The median time to lung specific recurrence was calculated as 13 months in the low-expression group, compared to 17 months in the high-expression group (P=0.096, log-rank test). The median time to tumor recurrence, irrespectively of the site of relapse, showed a significant difference between the low-expression (11 months) and the high-expression (17 months) group, respectively (P=0.041, log rank test). Overall survival in the low-expression group was 31 months, compared to 28 months in the high-expression group (P=0.055, log rank). COX-2, PGE2 and previously published prognostic factors of PM were included in a multivariate analysis (cut-off of P<0.2 in the univariate analysis). Neither COX-2 nor PGE were independent prognostic factors. However, the KRAS mutational status and the presence of lymphatic vessel invasion were associated with an impaired prognosis, as previously published (17,18).
Full table
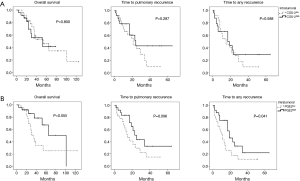
No correlation of stromal COX-2 and PGE2 expression levels and clinical outcome
We further evaluated the impact of stromal expression of COX-2 and PGE2 in pulmonary metastases specimens on clinical outcome after PM. Kaplan Meier curves for stromal COX-2 and PGE2 expression are presented in Figure 4. Comparable to the data on tumoral COX-2 and PGE2 expression, there was a trend towards a better outcome in the high expression group. However, this trend did not reach the level of significance in univariate and multivariate analysis using log-rank tests (Table 3).
Conclusions
PM is an integral part of the treatment of oligometastatic stage IV CRC cancer. By removing all evident tumor spread PM represents a potential curative treatment. Published series of PM for CRC described 5-year survival rates ranging from 40% to 68% (7). Despite these encouraging overall survival rates, there is a broad distribution of outcomes varying from long-term remission to tumor recurrence within several weeks (19). Traditional selection criteria of PM fail to identify patients with an impaired overall prognosis, who would only marginally benefit from a local resection of pulmonary nodules. Thus, attempts have been made to focus on tumor biology when selecting patients for PM. Kawaguchi et al. proposed an observation period with a repeat CT-scan calculating the tumor growth before PM. A tumor-doubling time of >100 days led to an increased recurrence free survival with a hazard ratio of 5.89 (1.89–18.32) in patients without preoperative chemotherapy (20). In addition to that a variety of molecular markers reflecting tumor aggressiveness have been proposed such as B-cell lymphoma 2 (bcl-2), β-catenin, carcinoembryonic antigen (CEA), E-cadherin, excision repair cross-complementation group 1 (ERCC1), KRAS, lymphatic invasion, CD34, pleural invasion, vascular invasion and vascular endothelial growth factorα (VEGFα) (9). None of those markers has been implicated in the clinical practice due to either technical difficulties or lack of standardization.
Inflammation is nowadays recognized as a major contributing factor to tumor growth and progression. Several inflammatory mediators have been linked to cancer progression such as VEGF-A, CSFs, IL-1, IL-6, IL-8, or CXCL1 (21,22). On the other hand, the immune system is also considered as one of the key factors of the endogenous cancer defence. In CRC the density of tumor infiltrating mature T-cells [cluster of differentiation (CD) 3+], cytotoxic T-cells (CD8+) and memory-T-cells (CD45RO+) are strong positive prognostic factors (23). By modifying the local immune reaction a tumor can escape this anti-tumor activity. Manipulating this process of immunoediting is an essential factor in the development of immune checkpoint blocking compounds (24).
COX-2 is the inducible isoform of the cyclooxygenase enzyme family. It is responsible for the conversion of arachidonic acid into prostaglandins. COX-2 is upregulated in inflammatory processes as well as in cancerous tissue (25). Preclinical studies on COX-2 as a possible therapeutic target in CRC were promising. Depletion of COX led to an increased anti-cancer immune response and T cell-mediated tumor elimination in experimental mouse models of CRC. Moreover, COX inhibitors enhanced the efficacy of immunotherapy with anti-PD-1 blocking antibodies (26). Although there is clear evidence that COX-2 is involved in tumor development and progression, its role as a prognostic marker is still elusive. This has recently been highlighted in a metanalysis including 18 studies on primary CRC. The hazard ratio for overall survival was only 1.19 and the impact on disease free interval was not significant. In addition to that the majority of included publications found only an indeterminate association of COX and patient prognosis. Of note most studies comprised a mixture of primary CRC patients and no analysis on the prognostic role of COX-2 in pulmonary metastases (27). One has to be careful when transferring conclusions from primary tumors to metastasized tumor stages. Metastases are distinct from their primary with a diverse tumor biology. It is generally believed that metastases acquire a more aggressive tumor phenotype during metastasis.
COX-2 expression in published series of primary CRC ranged from 33 percent to 84 percent (28,29). In our study cohort of metastatic CRC COX-2 could be detected in nearly all of our cases using an anti-COX-2 clone CX-294 antibody. This antibody is highly selective to detect COX-2 in formalin fixed tissue samples and was shown to have excellent staining characteristics (30). The high rate of COX-2 staining in our patient cohort might also reflect the advanced tumor stage and an unfavourable tumor biology of our patients. More than half of our patients had positive lymph nodes and almost all were in stage T3/4 at the time of diagnosis.
The role of PGE2 in colorectal cancer is manifold and it impacts the function of cancer cells as well as the function of almost all immune effector cells. In azoxymethane (AOM) mouse models PGE2 treatment significantly increased colon tumor incidence and promoted metastasis (31,32). PGE2 directly binds to the cell surface of CRC tumor cells and mediates anti-apoptosis, migration and invasion. Dependent on the binding to different prostanoid receptor types PGE-2 can act as both pro- and anti-inflammatory. As a pro-inflammatory mediator it regulates the expression profile of dendritic cells and enhances T cell activation (33). This is especially important in CRC, a tumor type considered highly immunogenic. A high number of tumor infiltrating CD8+ cells can induce a potent tumor lytic response and were shown to be prognostic in primary and metastatic CRC (34,35). On the other hand PGE2 can promote the formation of inhibitory T-regs in a cancerous environment (36). The number of tumor infiltrating T-regs are thought to be a negative prognostic factor in CRC. The herein reported findings in CRC pulmonary metastases suggest that high-expression of PGE2 in pulmonary metastases tissues reflects a beneficial tumor biology and leads to a longer disease free interval after PM.
The role of site specific recurrence after metastasectomy for CRC has recently been a focus of research. It seems that a certain tumor biology determines the site of recurrence. KRAS mutations for example are associated with increased lung metastasis, whereas loss of Smad4 expression seems to predict liver metastasis (17,37,38). This is an important information since repeated pulmonary and hepatic metastasectomy can be offered to the patients with good results and low morbidity (39). We therefore determined the site-specific pattern of metastatic recurrence in our patients. Both, COX-2 and PGE2 overexpression did not point towards a predominance in pulmonary recurrence.
The herein reported study collective is based on a prospectively documented data base introduced in 2009 at our institution. Since that time 53 patients with CRC pulmonary spreading, who underwent curative metastasectomy, were included in the data base. This study was designed as a pilot study to evaluate the frequency and degree of COX-2 and PGE2 expression in pulmonary metastases from CRC and to assess its impact on outcome data. Although a prognostic trend was observed for COX-2 and PGE2 in most survival/follow-up calculations, it did not reach the level of statistical significance. We currently recruit patients within an international multi-institutional study protocol in order to evaluate the impact of proposed prognostic markers (e.g., COX-2 and PGE2) in a larger cohort.
In conclusion, this pilot study shows that COX-2 and PGE2 are uniformly overexpressed in pulmonary metastases from CRC. High expression of COX-2 and PGE2 seem to reflect a beneficial tumor biology with late tumor recurrence and prolonged overall survival after PM. Further studies are warranted to confirm these findings in a larger study cohort.
Acknowledgements
Funding: This work was supported by a research grant (#15880) provided by the Austrian Federal Bank (OeNB) and by the Medical Scientific Fund of the Mayor of the City of Vienna (#15086).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of the Medical University of Vienna (EK#: 1097/2014) and was performed according to the Declaration of Helsinki and the Good Scientific Practice guidelines of the Medical University of Vienna.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [published 2013, accessed on 07 May 2016.] Available online: http://globocan.iarc.fr
- Hwang MR, Park JW, Kim DY, et al. Early intrapulmonary recurrence after pulmonary metastasectomy related to colorectal cancer. Ann Thorac Surg 2010;90:398-404. [Crossref] [PubMed]
- Rotolo N, De Monte L, Imperatori A, et al. Pulmonary resections of single metastases from colorectal cancer. Surg Oncol 2007;16 Suppl 1:S141-4. [Crossref] [PubMed]
- Nitsche U, Maak M, Schuster T, et al. Prediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collective. Ann Surg 2011;254:793-800; discussion 800-1. [Crossref] [PubMed]
- Vargas GM, Sheffield KM, Parmar AD, et al. Trends in treatment and survival in older patients presenting with stage IV colorectal cancer. J Gastrointest Surg 2014;18:369-77. [Crossref] [PubMed]
- Kim SK, Lee CH, Lee MR, et al. Multivariate Analysis of the Survival Rate for Treatment Modalities in Incurable Stage IV Colorectal Cancer. J Korean Soc Coloproctol 2012;28:35-41. [Crossref] [PubMed]
- Pfannschmidt J, Hoffmann H, Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J Thorac Oncol 2010;5:S172-8. [Crossref] [PubMed]
- Pastorino U. Lung metastasectomy: why, when, how. Crit Rev Oncol Hematol 1997;26:137-45. [Crossref] [PubMed]
- Schweiger T, Lang G, Klepetko W, et al. Prognostic factors in pulmonary metastasectomy: spotlight on molecular and radiological markers. Eur J Cardiothorac Surg 2014;45:408-16. [Crossref] [PubMed]
- Ghanim B, Schweiger T, Jedamzik J, et al. Elevated inflammatory parameters and inflammation scores are associated with poor prognosis in patients undergoing pulmonary metastasectomy for colorectal cancer. Interact Cardiovasc Thorac Surg 2015;21:616-23. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994;107:1183-8. [Crossref] [PubMed]
- Sano H, Kawahito Y, Wilder RL, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 1995;55:3785-9. [PubMed]
- Kargman SL, O'Neill GP, Vickers PJ, et al. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res 1995;55:2556-9. [PubMed]
- Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10:181-93. [Crossref] [PubMed]
- Schweiger T, Kollmann D, Nikolowsky C, et al. Carbonic anhydrase IX is associated with early pulmonary spreading of primary colorectal carcinoma and tobacco smoking. Eur J Cardiothorac Surg 2014;46:92-9. [Crossref] [PubMed]
- Schweiger T, Hegedüs B, Nikolowsky C, et al. EGFR, BRAF and KRAS status in patients undergoing pulmonary metastasectomy from primary colorectal carcinoma: a prospective follow-up study. Ann Surg Oncol 2014;21:946-54. [Crossref] [PubMed]
- Schweiger T, Nikolowsky C, Graeter T, et al. Increased lymphangiogenesis in lung metastases from colorectal cancer is associated with early lymph node recurrence and decreased overall survival. Clin Exp Metastasis 2016;33:133-41. [Crossref] [PubMed]
- Schweiger T, Starkl V, Glueck O, et al. Clinical impact of c-MET expression and mutational status in patients with colorectal cancer lung metastases. Eur J Cardiothorac Surg 2016;49:1103-11; discussion 1111. [Crossref] [PubMed]
- Kawaguchi K, Uehara K, Nakayama G, et al. Growth rate of chemotherapy-naïve lung metastasis from colorectal cancer could be a predictor of early relapse after lung resection. Int J Clin Oncol 2016;21:329-34. [Crossref] [PubMed]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211-7. [Crossref] [PubMed]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 2013;339:286-91. [Crossref] [PubMed]
- Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res 2014;20:1891-9. [Crossref] [PubMed]
- Page DB, Postow MA, Callahan MK, et al. Immune modulation in cancer with antibodies. Annu Rev Med 2014;65:185-202. [Crossref] [PubMed]
- Méric JB, Rottey S, Olaussen K, et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol 2006;59:51-64. [Crossref] [PubMed]
- Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015;162:1257-70. [Crossref] [PubMed]
- Peng L, Zhou Y, Wang Y, et al. Prognostic significance of COX-2 immunohistochemical expression in colorectal cancer: a meta-analysis of the literature. PLoS One 2013;8:e58891. [Crossref] [PubMed]
- de Heer P, Gosens MJ, de Bruin EC, et al. Cyclooxygenase 2 expression in rectal cancer is of prognostic significance in patients receiving preoperative radiotherapy. Clin Cancer Res 2007;13:2955-60. [Crossref] [PubMed]
- Wu AW, Gu J, Ji JF, et al. Role of COX-2 in carcinogenesis of colorectal cancer and its relationship with tumor biological characteristics and patients' prognosis. World J Gastroenterol 2003;9:1990-4. [Crossref] [PubMed]
- Habib A, Créminon C, Frobert Y, et al. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J Biol Chem 1993;268:23448-54. [PubMed]
- Wang D, Fu L, Sun H, et al. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology 2015;149:1884-1895.e4. [Crossref] [PubMed]
- Kawamori T, Uchiya N, Sugimura T, et al. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis 2003;24:985-90. [Crossref] [PubMed]
- Krause P, Bruckner M, Uermösi C, et al. Prostaglandin E(2) enhances T-cell proliferation by inducing the costimulatory molecules OX40L, CD70, and 4-1BBL on dendritic cells. Blood 2009;113:2451-60. [Crossref] [PubMed]
- Remark R, Alifano M, Cremer I, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 2013;19:4079-91. [Crossref] [PubMed]
- Schweiger T, Berghoff AS, Glogner C, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin Exp Metastasis 2016;33:727-39. [Crossref] [PubMed]
- Baratelli F, Lin Y, Zhu L, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 2005;175:1483-90. [Crossref] [PubMed]
- Lipsyc M, Yaeger R. Impact of somatic mutations on patterns of metastasis in colorectal cancer. J Gastrointest Oncol 2015;6:645-9. [PubMed]
- Losi L, Bouzourene H, Benhattar J. Loss of Smad4 expression predicts liver metastasis in human colorectal cancer. Oncol Rep 2007;17:1095-9. [PubMed]
- Park JS, Kim HK, Choi YS, et al. Outcomes after repeated resection for recurrent pulmonary metastases from colorectal cancer. Ann Oncol 2010;21:1285-9. [Crossref] [PubMed]


