Survival rate and prognostic factors of surgically resected clinically synchronous multiple primary non-small cell lung cancer and further differentiation from intrapulmonary metastasis
Introduction
The detection rate of multiple pulmonary nodules is increasing rapidly. Clinicopathological assessment, diagnosis, and management have evolved, but remain severely limited. Multiple primary lung cancer (MPLC) is defined as primary lung cancer occurring simultaneously or sequentially in different areas in unilateral or bilateral lungs. The criteria were first proposed by Martini and Melamed (1) in 1975, which categorize MPLC into synchronous multiple primary (SMP) lung cancer and metachronous MPLC occurring within a 2-year interval. The revised diagnostic criteria proposed by the American College of Chest Physicians (ACCP) have been commonly applied for clinical diagnosis since 2003 (2-4). The distinction of intrapulmonary metastases from independent primary tumors is difficult but of great clinical importance as it influences staging and potentially the therapeutic strategy. Here we studied a cohort of 52 patients with clinically SMP non-small cell lung cancer (SMP-NSCLC) and performed a survival analysis aiming to clarify diagnostic and treatment strategies. We also assessed the possibility of screening SMP-NSCLC through comprehensive histologic assessment and next generation sequencing (NGS).
Methods
Ethics statement
This retrospective study was performed after having been approved by the ethics committee of the China-Japan Friendship Hospital (2013-MS-076). The methods were carried out in accordance with the relevant guidelines and regulations, and written informed consent was given by the patients for their information to be stored in the hospital database and used for clinical research.
Patients and clinical features
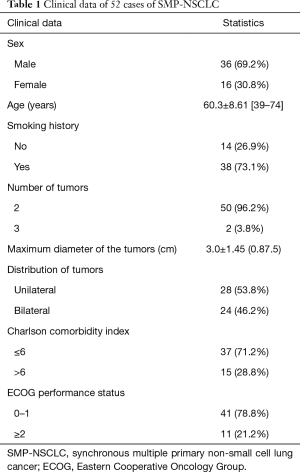
Between November 2004 and December 2015, 52 patients were diagnosed as having SMP-NSCLC according to the Martini-Melamed criteria (1). All patients underwent surgical resections in the Department of Thoracic Surgery, China-Japan Friendship Hospital among 3,527 patients (accounting for 1.5%) who were treated for lung cancer during that period. Of these, 20 patients were diagnosed as having SMP-NSCLC of the same histological type, including 18 cases of multiple primary lung adenocarcinoma. Thirty-two patients were diagnosed as having two different histological types. Carcinoid tumors, lung sarcomatoid carcinomas and other rare tumors were excluded from the study.
Positron emission tomography-computed tomography (PET-CT) was performed in 28 cases while mediastinoscopy or endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) was performed in 24 cases preoperatively to exclude those clinical/pathological N2 stage cases. All of the 106 nodules from 52 cases were surgically resected. Detailed clinical information is shown in Table 1.
Full table
All patients underwent a bronchoscopy before the operation; cranial CT/magnetic resonance imaging (MRI), abdominal ultrasonography/CT, and a nuclide bone scan were adopted to rule out extrapulmonary metastasis before PET-CT was widely adopted. The growing use of PET-CT provided an effective choice for the evaluation of pulmonary nodules before surgery. Of the 28 unilateral SMP-MPLC cases, 6 were diagnosed via CT-guided transthoracic fine needle aspiration biopsy (TTFNAB) before surgery, 19 were diagnosed by fast frozen pathology during the surgical procedure, and 3 were diagnosed by postoperative pathology. Of the 24 bilateral MPLC cases, 4 were diagnosed by CT-guided TTFNAB before surgery and 20 were diagnosed by pathology during or after the surgical procedure. For patients with lower-limit pulmonary function, preoperative examinations, such as blood gas analysis, a stairwell test and a 6-minute walking test, were arranged as necessary.
Surgical procedures
Considering the patient’s age and cardiopulmonary function, in association with the size, quantity, and distribution of tumors, different surgical procedures were adopted according to the experience of the surgeons, which included the following:
- Anatomical lobectomy and systemic mediastinal lymph node dissection, applicable to multiple tumors in one lobe;
- Anatomical bilateral lobectomies (or combined lobectomies) and systemic mediastinal lymph node dissection, applicable to patients with multiple tumors in bilateral lobes (or two right lobes) with tolerable cardiopulmonary function;
- Anatomical lobectomy together with sublobar resection (anatomical segmentectomy/wedge resection) and systemic mediastinal lymph node dissection, applicable to multiple tumors occurring unilaterally or bilaterally. While a larger lesion is eligible for lobectomy, a smaller one is located at the edge of the lung with a diameter of less than 2 cm;
- Multiple sublobar resection and mediastinal lymph node sampling, applicable to multiple tumors all located at the edge of the lung with a diameter less than 2 cm, or elderly patients not eligible for lobectomy owing to their poor cardiopulmonary function;
- Total pneumonectomy and systemic mediastinal lymph node dissection, applicable to tumors located in more than one lobe unilaterally, or the central types that cause difficulty in performing a lobectomy even though cardiopulmonary function could tolerate surgery.
Video-assisted thoracoscopic surgery (VATS) was first considered in all cases wherein the lesion was large or dissection during the operation was difficult; alternatively, open thoracotomy was adopted. Wedge resection and anatomical segmentectomy should ensure that the distance between the margin of the resected lung parenchyma and the margin of the tumor was not less than the tumor’s maximum diameter or more than 2 cm. Wedge resection was commonly applied previously. With improvements in surgical technique, anatomical segmentectomy is widely adopted at present. For bilateral SMP-NSCLC, the time-window for the second operation was chosen according to the surgeon’s experience, considering the patient’s general condition and cardiopulmonary function. Of the 24 bilateral cases, 18 involved synchronous bilateral procedures, while 6 cases involved procedures within a 4–10 week interval. Before the second procedure, a chest CT scan and re-evaluation of the pulmonary function were required. For patients with restricted pulmonary function, sublobar resection was recommended. Adjuvant chemotherapy was recommended to postoperative patients, except for those pathologically diagnosed with multiple T1N0M0, IA stage NSCLC. A combination chemotherapy regimen with platinum was regularly adopted (5,6).
Pathological diagnosis
All of the 106 tumors were surgically resected and received a definite pathological diagnosis. If tumors were of the same pathological type, the lymph node metastasis status was required to determine whether there was metastasis in mutual lymphatic drainage regions according to the Martini-Melamed criteria.
Considering the latest revised diagnostic criteria proposed by ACCP in 2013 (4), pathological subtypes of the 18 cases of multiple primary lung adenocarcinoma were identified according to the international multidisciplinary classification of lung adenocarcinoma (7). Lung adenocarcinoma was classified as pre-invasive lesion such as atypical adenomatous hyperplasia and adenocarcinoma in situ, minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma classified by a predominant pattern after using comprehensive histologic subtyping with lepidic, acinar, papillary, micropapillary and solid patterns. Variants of invasive adenocarcinomas were included as well.
NGS was applied to 6 cases of multiple primary lung adenocarcinomas with similar pathological subtypes for further differentiation from intrapulmonary metastasis. Semiconductor sequencing based on the Ion Personal Genome Machine (PGM™) System (Thermo Fisher Scientific, USA) was performed with the Ion AmpliSeq Cancer Panel v2 (Thermo Fisher Scientific, USA) to sequence more than 2,800 loci from 50 oncogenes and tumor suppressor genes in tumor DNA.
Follow up examination
All patients received regular follow-up. The median follow-up time was 43 months (range from 6 to 88 months). Chest CT and serum tumor markers (CEA, cyfra21-1, SCC, CA199, and CA125) were examined every 3 months. Cranial CT/MRI, abdominal ultrasonography/CT, and nuclide bone scans were performed every half year, and whenever necessary with positive symptoms. The starting point of follow-up was defined as the date of surgery or second surgery, while death was defined as the end point.
Statistical analysis
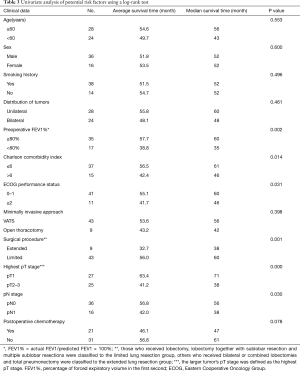
SPSS 19.0 software (IBM, Chicago, USA) was used for statistical analysis. Kaplan–Meier estimator was used to draw survival curves. Potential risk factors such as patient’s age, sex, smoking history, distribution of tumors, preoperative percentage of forced expiratory volume in the first second (FEV1%, defined as actual FEV1/predicted FEV1 × 100%), the Charlson comorbidity index, Eastern Cooperative Oncology Group (ECOG) performance status, a minimally invasive approach and different surgical procedures, highest pT stage, pN stage and postoperative adjuvant chemotherapy were subject to a univariate analysis using a log-rank test. A Cox proportional hazards model was adopted to perform a multivariate analysis on the potential above mentioned risk factors to identify independent risk factors. Significance was defined as P<0.05.
Results
The surgical procedures and initial pathological results for all 52 cases of SMP-MPLC are shown in Table 2.

Full table
According to the 7th edition of TNM staging for NSCLC, if the larger tumor’s pT stage was defined as the highest pT stage, 27 cases would be classified as pT1, while there were 24 cases of pT2 and 1 case of pT3. Thirty-six cases were confirmed as pN0, while the other 16 cases were confirmed as pN1. Postoperative adjuvant chemotherapy was applied to 6 of 12 pT2-3N0M0 cases and 15 of 16 pN1 cases.
Three cases (5.8%) suffered postoperative complications, including 1 case (a 52-year-old man, with adenocarcinoma on the right upper lobe together with squamous cell carcinoma on the left lower lobe, received synchronous bilateral lobectomies) who required sustained assistance with a mechanical ventilator due to postoperative hypoxemia. He was successfully detached from the respirator after a long term of respiratory function exercises. Two cases sustained pulmonary air-leakage and recovered after applying negative pressure suction.
No perioperative mortality was observed.
General survival rate and prognostic factors
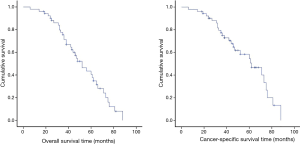
The Kaplan-Meier overall survival times and cancer-specific survival curve are shown in Figure 1. The overall 5-year survival rate of the 52
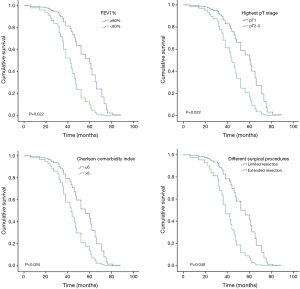
Patients were divided into groups according to age, sex, smoking history, distribution of tumors, preoperative FEV1%, Charlson comorbidity index, ECOG performance status, a minimally invasive approach, different surgical procedures, highest pT stage, pN stage, and postoperative adjuvant chemotherapy. A log-rank test was used for univariate statistical analysis and the results are shown in Table 3. Preoperative FEV1% ≥80% (P=0.002), Charlson comorbidity index ≤6 (P=0.014), ECOG performance status =0 or 1 (P=0.031), limited extent of resection (P=0.001), pT1 (P=0.000) and pN0 (P=0.030) might be potential indicators of a better prognosis.
Full table
For the purpose of further eliminating the interactions of variants, a Cox proportional hazards model was used to perform multivariate analyses on potential risk factors (Table 4). Preoperative FEV1% (P=0.022), Charlson comorbidity index (P=0.034), different surgical procedures (P=0.040) and highest pT stage (P=0.022) were identified as independent risk factors for overall survival.
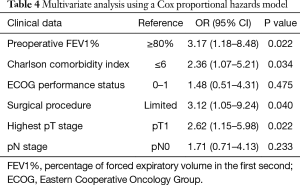
Full table
Cox regression overall survival curves drawn from the differences of preoperative FEV1%, Charlson comorbidity index, surgical procedure and highest pT stage are shown in Figure 2.
Further differentiation from intrapulmonary metastasis
Pathological subtypes of the 18 cases of clinically multiple primary lung adenocarcinomas were identified according to the international multidisciplinary classification (7). Detailed results are shown in Table 5.
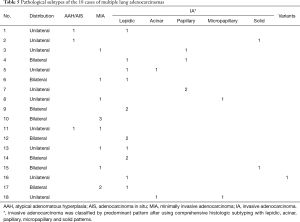
Full table
Patients 1–3 underwent lobectomy, patient 4 underwent bilateral lobectomies, patient 5 underwent combined lobectomies, patients Nos. 6–7 underwent lobectomy together with wedge resection, patients Nos. 8–9 underwent lobectomy together with segmentectomy, patient 10 underwent three wedge resections, patients 11–13 underwent two wedge resections, patients 14–15 underwent two segmentectomies, patient 16 underwent segmentectomy together with wedge resection, patient 17 underwent segmentectomy together with two wedge resections and patient 18 underwent left total pneumonectomy.
As similar pathological subtype of adenocarcinoma was identified among tumors of patients 7, 9, 10, 12, 14, and 17. Further differentiation from intrapulmonary metastasis was performed under NGS. Detailed results are shown in Table 6.
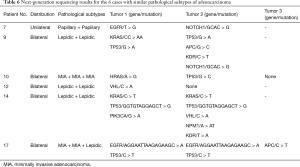
Full table
Discussion
Clinical guidelines have been developed to distinguish independent NSCLCs from metastases. Currently, the diagnosis of SMP-NSCLC mainly relies on the Martini-Melamed criteria: (I) histologically, all lesions are malignant; (II) all lesions exist independently; (III) the possibility of inter-metastasis is excluded; and (IV) If the histologic diagnosis is the same, simultaneous lesions must be located in different segments/lobes with no lymph node or extrapulmonary metastasis. In fact, the diagnosis of MPLC is easier to obtain when two tumors are of different pathological types. For tumors with the same pathological diagnosis, such as two adenocarcinomas, a differential diagnosis with intrapulmonary metastasis is difficult, and there is a lack of a preoperative diagnostic method in particular.
In the 7th edition of TNM staging for NSCLC, SMP lung cancer and metastases are not clearly distinguished. It is known that metastatic cancer has a poor prognosis (8); therefore, systemic adjuvant chemotherapy or targeted therapy is the main clinical treatment. For SMP-NSCLC patients, definite clinical/pathological staging remains unclear, although in the forthcoming 8th edition of TNM staging, it is proposed that for multifocal ground glass/lepidic tumors, the T category be determined by the highest T lesion, and that a single N and M category can be used for all lesions collectively, for example, T1a(3)N0M0 or T1b(m)N0M0. Only for diffuse pneumonic-type lung cancer, is it proposed that the T category be designated by size (or T3) if in one lobe, as T4 if involving an ipsilateral different lobe, or as M1a if contralateral (9). Positive surgical treatment could benefit the prognosis, according to the previous literature or our experience. The postoperative median survival time of SMP-NSCLC could be more than 60 months (10,11). According to the international multidisciplinary classification of lung adenocarcinoma (7), the 5-year survival rate after radical resection of an adenocarcinoma in situ and MIA could reach nearly 100%, making these types of lung adenocarcinomas close to curable (12-14). For SMP-NSCLC that presents as multiple ground–glass-nodules (GGNs, mostly preinvasive lesions/MIAs), the 3-year survival rate after radical resection was reported to be as high as 92.9% (15), which is inclined to be classified as early stage lung cancer, with significant deference to metastases. Even after excluding tumors with a better prognosis, such as multiple primary GGN-like adenocarcinomas, carcinoid tumors, and satellite nodules, previous studies demonstrated that the therapeutic effect of SMP-NSCLC was satisfactory with a postoperative 5-year survival rate of nearly 50% (16,17).
The overall 5-year survival rate of the 52 SMP-NSCLC patients in our cohort was 40.6%, and the postoperative median survival time was 52 months, which were slightly lower than the previous reports mentioned above. Although the cancer-specific 5-year survival rate in our cohort could reach 54.5%, it was believed that the main cause of the low survival rate in our cohort might be relatively advanced staging of lung cancer, as nearly half the patients (25/52) were discovered with at least one pT2 lesion and nearly one third of them were classified as pN1, while only a dozen would be classified as to multiple GGNs.
In univariate analysis of the prognostic factors of SMP-NSCLC, ≥60 years old (P=0.553), sex (P=0.600), smoking history (P=0.496), distribution of tumors (P=0.461), minimally invasive approach (P=0.398) and postoperative adjuvant chemotherapy (P=0.078) did not lead to a statistical difference between the groups. Preoperative FEV1% (P=0.002), Charlson comorbidity index (P=0.014), ECOG performance status (P=0.031), different surgical procedures (P=0.001), highest pT stage (P=0.000) and pN stage (P=0.030) were identified as potential risk factors. Advanced age or smoking history might not be an absolute contradiction for surgery for SMP-NSCLC. Unilateral and bilateral SMP-NSCLC patients had approximately the same prognosis, indicating similar staging, and both categories should not be treated differently in the view of “extent of metastasis.” Aggressive treatment might improve the prognosis for carefully selected patients with preoperative evaluation of the general condition and cardiopulmonary function. Applying VATS or postoperative adjuvant chemotherapy would not significantly improve the prognosis.
For further eliminating the interaction of variants, multivariate analysis was performed using a Cox proportional hazards model, and preoperative FEV1% (P=0.022), Charlson comorbidity index (P=0.034), different surgical procedures (P=0.040) and highest pT stage (P=0.022) were confirmed as independent risk factors affecting the prognosis of SMP-NSCLC patients after surgical resection. Although such statistical analyzing method still cannot rule out selection bias completely due to the limited number of enrolled cases and the specific characters of SMP-NSCLC, this result again confirmed the importance of preoperative evaluation of comorbidity conditions and pulmonary function, which has been clarified in previous literature (18-20). The prognosis of patients who received extended resection (total pneumonectomy and bilateral or combined lobectomies) was significantly worse than in those who received a limited resection (single lobectomy, lobectomy together with sublobar resection and multiple sublobar resections). This indicated the importance of avoiding a so-called “radical resection” in the treatment of SMP-NSCLC. The general consensus was that pneumonectomy had the highest complication and mortality rate of all elective pulmonary resections. A study based on the National Cancer Database indicated that the overall 30-day mortality rate for pneumonectomy in the treatment of NSCLC was 8.5%, which was far above the average level (21), while the predicted postoperative forced expiratory volume in 1 second may be useful for identifying high risk patients for pulmonary complication development and adverse outcomes (22). The prognosis of SMP-NSCLC patients with multiple pT1 tumors was far better than those with pT2/pT3 tumors, which was consistent with previous reports (11,23) and clinical experience. The result of multivariate analysis indicated that pN stage might not be the direct influential factor for the prognosis of SMP-NSCLC. Enlargement of tumor size is often associated with lymph node metastasis, while the highest pT stage should take the leading role in evaluating the prognosis. It should be noted that the limited sample size and relatively lower intrapulmonary lobar and segmental lymph node dissection rate might have a negative impact on this result. An expanded sample size and more rigorous lymph node dissection might reconfirm the effect of pN stage in evaluating the prognosis of SMP-NSCLC patients.
As mentioned above, the overall 5-year survival rate and postoperative median survival time in our cohort were slightly lower than the previous reports (16,17). On one hand, it might be due to relatively advanced staging of lung cancer in our cohort, as mentioned above. On the other hand, Martini-Melamed criteria for MPLC is based on clinical characteristics, which are subjected to the limitation of lymph node dissection area and false negative results of common lymphatic drainage areas that could lead to difficulty distinguishing between intrapulmonary metastasis and MPLC. The more effective way might rely on further differentiation of pathological subtypes or a molecular pathological diagnostic method, as discussed and introduced in the latest ACCP diagnostic criteria (4). In our cohort, different pathological subtypes were identified in 13 of the 18 cases of multiple lung adenocarcinomas, making the detection rate as high as 72.2%, while similar results from Girard et al. (24) also indicated that comprehensive histologic assessment would be a powerful tool to determine whether multiple lung adenocarcinomas or squamous cell carcinomas are metastatic or multiple primaries. The remaining 5 cases had consistent pathological subtypes and 1 case (patient 17) with 2 nodules of the same pathological subtype. Considering that different lung cancers in the same individual may have distinct genomic profiles and can be driven by distinct molecular events (25), semiconductor sequencing based on the Ion Torrent Personal Genome Machine (PGM) was performed with the Ion AmpliSeq Cancer Panel v2 to detect more than 2,800 hotspot mutations in 50 oncogenes and tumor suppressor genes in tumor DNA. As shown in Table 6, patients 7, 10, and 12 had completely different mutations. Patients 9 and 14 each shared one consistent mutation in TP53, but were both identified as having different mutations between tumors at the same time. Although patient 17 had already been identified as having multiple primary lung adenocarcinomas with different pathological subtypes of adenocarcinomas, both MIAs had the same mutation on EGFR and TP53, which was different from the mutations detected in the other lepidic predominant invasive adenocarcinomas.
It was reported that the discrimination rate of genotypes for MPLC could reach 86.2% when performing combined detection of p53/EGFR mutations (26), which might help in estimating the relationship between multiple tumors. A diagnostic lineage test based on genomic rearrangements from mate-pair sequencing has previously been applied for distinguishing independent primary from metastatic lung cancers (27); therefore, we believe that a single or a few differences in gene mutations are not sufficient to confirm the presence of two malignent lesions from different sources based on the awareness of genetic heterogeneity in non-small-cell lung cancers (28). NGS and the continuing upward trajectory of sequencing technology development is enabling clinical applications that are aimed at improving medical diagnosis and treatment (29). Future research could focus on identifying a proper genetic locus and detection method with satisfactory sensitivity and specificity to accurately differentiate SMP-NSCLC from intrapulmonary metastasis.
Acknowledgements
Funding: Fei Xiao M.D. received funding from the China-Japan Friendship Hospital Youth Science and Technology Excellence Project (2014-QNYC-B-09).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was performed after having been approved by the ethics committee of the China-Japan Friendship Hospital (2013-MS-076). The methods were carried out in accordance with the relevant guidelines and regulations, and written informed consent was given by the patients for their information to be stored in the hospital database and used for clinical research.
References
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Detterbeck FC, Jones DR, Kernstine KH, et al. American College of P. Lung cancer. Special treatment issues. Chest 2003;123:244S-58S. [Crossref] [PubMed]
- Shen KR, Meyers BF, Larner JM, et al. American College of Chest P. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:290S-305S.
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003;21:3016-24. [Crossref] [PubMed]
- Kreuter M, Vansteenkiste J, Fischer JR, et al. Randomized phase 2 trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: the TREAT study. Ann Oncol 2013;24:986-92. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Otani S, Sato Y, Endo S, et al. Prognosis of patients after resection for lung cancer with intrapulmonary metastasis in different lobes. Kyobu Geka 2006;59:26-30. [PubMed]
- Detterbeck FC, Marom EM, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Application of TNM Staging Rules to Lung Cancer Presenting as Multiple Nodules with Ground Glass or Lepidic Features or a Pneumonic Type of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol 2016;11:666-80.
- Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. [Crossref] [PubMed]
- Tanvetyanon T, Robinson L, Sommers KE, et al. Relationship between tumor size and survival among patients with resection of multiple synchronous lung cancers. J Thorac Oncol 2010;5:1018-24. [Crossref] [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [Crossref] [PubMed]
- Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int 2012;62:785-91. [Crossref] [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. [Crossref] [PubMed]
- Kocaturk CI, Gunluoglu MZ, Cansever L, et al. Survival and prognostic factors in surgically resected synchronous multiple primary lung cancers. Eur J Cardiothorac Surg 2011;39:160-6. [Crossref] [PubMed]
- Fabian T, Bryant AS, Mouhlas AL, et al. Survival after resection of synchronous non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;142:547-53. [Crossref] [PubMed]
- Nakajima T, Sekine Y, Yamada Y, et al. Long-term surgical outcome in patients with lung cancer and coexisting severe COPD. Thorac Cardiovasc Surg 2009;57:339-42. [Crossref] [PubMed]
- Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer 2011;73:237-42. [Crossref] [PubMed]
- Luchtenborg M, Jakobsen E, Krasnik M, et al. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur J Cancer 2012;48:3386-95. [Crossref] [PubMed]
- Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg 2014;98:1953-60. [Crossref] [PubMed]
- Algar FJ, Alvarez A, Salvatierra A, et al. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur J Cardiothorac Surg 2003;23:201-8. [Crossref] [PubMed]
- Tanvetyanon T, Finley DJ, Fabian T, et al. Prognostic factors for survival after complete resections of synchronous lung cancers in multiple lobes: pooled analysis based on individual patient data. Ann Oncol 2013;24:889-94. [Crossref] [PubMed]
- Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 2009;33:1752-64. [Crossref] [PubMed]
- Liu Y, Zhang J, Li L, et al. Genomic heterogeneity of multiple synchronous lung cancer. Nat Commun 2016;7:13200. [Crossref] [PubMed]
- Chang YL, Wu CT, Lin SC, et al. Clonality and prognostic implications of p53 and epidermal growth factor receptor somatic aberrations in multiple primary lung cancers. Clin Cancer Res 2007;13:52-8. [Crossref] [PubMed]
- Murphy SJ, Aubry MC, Harris FR, et al. Identification of Independent Primary Tumors and Intrapulmonary Metastases Using DNA Rearrangements in Non-Small-Cell Lung Cancer. J Clin Oncol 2014. [Crossref] [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. [Crossref] [PubMed]
- Mardis ER. A decade's perspective on DNA sequencing technology. Nature 2011;470:198-203. [Crossref] [PubMed]