T1 esophageal cancer, request an endoscopic mucosal resection (EMR) for in-depth review
Esophageal adenocarcinoma (EAC) has seen a dramatic increase in Europe, Australia and the United States over the last 30-40 years, whereas the rates of squamous cell cancer of the esophagus has remained relatively stable or decreasing in Western countries (1-3). In 2013, it is estimated that there will be 17,990 new diagnoses of esophageal cancer in the United States, with 15,210 patients who will die from this disease (4). In the United Kingdom and the United States, adenocarcinoma has become the dominant histologic form of cancer in the esophagus (5). Twenty percent of all EAC in the United States is early stage (T1) with disease confined to the mucosa or submucosa (6,7). Traditionally surgery has been the standard of care for early stage EAC. However, there is substantial morbidity (30-50%) associated with esophagectomies (8,9). In addition in-hospital mortality rates can be high as 8% in low-volume hospitals compared to 2-3% in high-volume institutions (10). A less invasive alternative to surgery for early stage EAC is endoscopic resection and ablation.
In early stage EAC the risk of lymph node metastasis correlates to the depth of involvement of the cancer (11). A large retrospective review of 126 T1 EAC, of which 75 were T1a and 51 T1b, revealed lymph node metastasis of 1.3% and 22% respectively (12). A more comprehensive subclassification of early esophageal cancer has been proposed with mucosal disease and submucosal disease divided into three categories respectively (m1-3, and sm1-3) based on depth of invasion (11,13). Data on superficial squamous cell cancer of the esophagus (SCCA) have shown that m3 cancer, or disease extending to the muscularis propria has upwards of 6% risk of LN metastasis (11). Additional characteristics which impact the risk of lymph node metastasis include lymphovascular invasion, size of the tumor, and the degree of tumor differentiation (11,12,14). Given the low risk of lymph node metastasis in mucosal disease, endoscopic management of early stage EAC is limited to disease confined to the mucosa (T1a).
Data on the efficacy of endoscopic therapy in early EAC is limited to case series with short follow-up duration. At present there is no randomized controlled trial comparing the outcomes between endoscopic therapy and surgery in the management of early stage EAC. Given the inherent challenge in trying to randomize between two radically different treatments, such a study will be difficult to accomplish. Current literature suggests that in appropriately selected patients with early stage EAC, endoscopic management does have comparable outcomes to surgery with fewer complications, but a higher rate of recurrence (15,16). A large retrospective cohort study which evaluated endoscopic resection in combination with photodynamic therapy compared to surgery showed comparable survival in patients with T1a EAC in Barrett’s esophagus (BE) (15). A second study examining endoscopic resection in combination with argon plasama coagulation of remaining non-dysplastic BE compared to transthoracic resection with 2-field lymphadenectomy in T1a EAC, showed similar rates of complete remission (16). The surgical group did have a higher morbidity and mortality, whereas the endoscopy group had a higher rate of metachronous lesions which were managed by endoscopy (16). There is emerging data showing that pT1b EAC with favorable characteristics (sm1, well-to-moderate tumor differentiation and no lymphovascular invasion) maybe potential candidates for endoscopic therapy for curative intent (17,18). With increasing data on the efficacy of endoluminal therapies, endoscopic resection and ablation have gained wider acceptance as an alternative to surgery in the management of early EAC (19). The key challenge for physicians is determining which patients are appropriate candidates for endoscopic therapy.
Endoscopic ultrasound (EUS) is an integral component in the locoregional staging of esophageal cancer. The overall T-stage accuracy of EUS improves with increase depth of invasion, with the highest accuracy seen in T4 tumors (88-100%) (20), however, there has been controversy surrounding the accuracy of EUS in the evaluation of early EAC. A meta-analysis of 12 studies showed that in comparison with surgical or endoscopic mucosal resection (EMR) pathology, EUS had T-stage concordance of only 65% in early EAC (21). A recent larger meta-analysis encompassing 19 studies with a total of 1,019 patients including EUS with radial probes and higher frequency mini-probes, showed EUS to have overall good accuracy in staging T1a and T1b esophageal cancers with area under the curve
A retrospective study of 131 patients evaluated if EUS changed the management approach of patients with early EAC (23). In this study 105 of the 131 patients had an unremarkable EUS. After EMR 17 patients were found to have submucosal invasion, 2 patients with positive deep resection margins and 6 with poorly differentiated cancer and/or lymphovascular invasion. Despite a normal EUS, after EMR, 25 out of the 105 patients had risk factors for lymph node metastasis that would have been missed without the corresponding histology. This study highlights the potential risk of “under-treatment”. Conversely in the 26 patients who had an EUS suggestive of submucosal invasion or lymph node metastasis, EMR revealed no risk factors for lymph node metastasis in 10 of these patients. Referral to surgery based on the EUS findings would have subjected these patients to “over-treatment”. In this series of patients with early EAC, EUS had no clinical impact on patient management.
EMR has grown in importance in the management of early EAC as both a diagnostic and therapeutic modality (Figure 1). A large single center study examining complete Barrett’s eradication EMR (CBE-EMR) for the management of BE with high grade dysplasia (HGD) and intramucosal cancer (IM) for curative intent reinforces the importance of EMR in early EAC (24). In this study a total of 49 patients with biopsy confirmed BE with HGD/IM underwent CBE-EMR for a total of 106 EMR sessions. Overall 32 patients were able to complete CBE-EMR and on surveillance 31 of 32 (96.9%) had normal squamous epithelium. In the pathologic comparison of the pre-EMR biopsy and the CBE-EMR, there was a 45% change in the final pathologic stage. 14% of patients were upstaged and an additional 31% were down staged. The upstaging of disease was likely due to the limited depth of the biopsy specimens whereas the down staging was attributable to the complete removal of the HGD/IM foci with biopsies. Furthermore, four patients were found to have advanced disease, two with submucosal invasion and two with lymphovascular invasion, requiring a referral to surgery. In this study, EMR provided accurate staging data in addition to providing information which affected patient management.
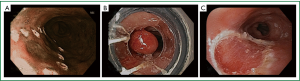
EMR as an endoscopic technique has been around for more than 20 years. It was initially described by Inoue using a cap technique for resection of early neoplastic lesions (25). A transparent cap with a circumferential rim where a diathermic snare can be loaded is fixed to the tip of the endoscope. After the neoplastic lesion is identified, the border of the lesion is marked with APC probe. Then saline is injected at the base of the lesion, providing a lift. Methylene blue can also be added to the saline solution to help differentiate the submucosa from the muscle layer. Next, the marked lesion is sucked into the distal attachment and the snare is used to grasp and ligate the lesion with electrocautery. En-bloc resection of up to 20 mm can be achieved with the cap technique.
An alternative to the EMR technique is the use of a reusable variceal band ligating device attached to the tip of the endoscope. The neoplastic lesion is sucked into the ligation cap, and then a rubber band is applied creating a pseudopolyp. The endoscope is withdrawn and the band ligating device is disassembled and standard polypectomy is performed on the pseudopolyp. With this technique submucosal injection of saline is not necessary.
A prospective randomized trial examined these two techniques in 72 patients with early EAC who underwent 100 endoscopic resections (26). Fifty endoscopic resections were performed with the cap technique with prior submucosal injection of a dilute saline solution of epinephrine, and 50 resections were performed with a “suck-and-ligate” device without prior submucosal injection. Both techniques were safe with no severe complications and only a minor case of bleeding in each group. With regards to size of the resected specimen, there was no statistically significant difference between the cap group and the ligation group (15.7 mm × 10.7 mm versus 16.4 mm × 11 mm).
Recently, EMR with the band ligating device has been supplanted by multiband mucosectomy (MBM). MBM uses a modified variceal band ligator containing six bands and allows passage of a snare through the biopsy channel of the endoscope. Up to six resections can be performed without the need to remove the endoscope between banding and polypectomy. A prospective study of MBM in 170 patients who underwent a total of 243 MBM procedures, with 1,060 resections demonstrated the safety and efficacy of this technique (27). Complete endoscopic resection was achieved in 91% of focal lesions. There were no perforations and delayed bleeding occurred in 2% of patients, all of whom were managed by endoscopy. A multicenter randomized control trial comparing endoscopic resection-cap technique and MBM for piecemeal endoscopic resection of early Barrett’s neoplasia found procedure time (34 vs. 50 min) and cost was significantly cheaper (euro 240 vs. euro 322) with MBM (28). MBM did result in smaller resection specimens than endoscopic resection-cap technique (18 mm × 13 mm vs. 20 mm × 15 mm), however, this was felt to be clinically insignificant as the depth of resection of both techniques were the same.
As the role of therapeutic endoscopy expands into the realm of early EAC, accurate staging is of utmost importance. Overestimating the T-stage may subject patients to surgery with high morbidity and a considerable mortality rate when it may have been managed by endoscopy. Underestimation of the T-stage may lead to insufficient treatment by endoscopy with potential lymph node metastasis being left untreated. EUS has played a significant role in the staging of EAC, however, EUS cannot definitively discriminate between mucosal and submucosal disease in early EAC. On the other hand, EMR is an established endoscopic technique, with a good safety profile. An EMR can provide clear detail on the depth of invasion in early EAC and further sub classify the disease (m1-3 and sm1-3). In addition, the histologic correlate from the EMR provides valuable information regarding risk factors for lymph node metastasis. For T1 EAC, EMR should be the final diagnostic step in determining whether a patient should undergo surgery or endoscopic therapy.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Edgren G, Adami HO, Weiderpass E, et al. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013. [Epub ahead of print]. [PubMed]
- Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013;119:1149-58. [PubMed]
- Vizcaino AP, Moreno V, Lambert R, et al. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer 2002;99:860-8. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Bennett C, Green S, Decaestecker J, et al. Surgery versus radical endotherapies for early cancer and high-grade dysplasia in Barrett’s oesophagus. Cochrane Database Syst Rev 2012;11:CD007334. [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [PubMed]
- Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340-5. [PubMed]
- Lagarde SM, Vrouenraets BC, Stassen LP, et al. Evidence-based surgical treatment of esophageal cancer: overview of high-quality studies. Ann Thorac Surg 2010;89:1319-26. [PubMed]
- Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424-9. [PubMed]
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg 2012;16:1055-63. [PubMed]
- Shimada H, Nabeya Y, Matsubara H, et al. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg 2006;191:250-4. [PubMed]
- Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. [PubMed]
- van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011;60:765-73. [PubMed]
- Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005;29:1079-85. [PubMed]
- Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815-23. [PubMed]
- Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg 2011;254:67-72. [PubMed]
- Manner H, Pech O, Heldmann Y, et al. Efficacy, Safety, and Long-term Results of Endoscopic Treatment for Early Stage Adenocarcinoma of the Esophagus With Low-risk sm1 Invasion. Clin Gastroenterol Hepatol 2013;11:630-5. [PubMed]
- Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008;103:2589-97. [PubMed]
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143:336-46. [PubMed]
- Saunders HS, Wolfman NT, Ott DJ. Esophageal cancer. Radiologic staging. Radiol Clin North Am 1997;35:281-94. [PubMed]
- Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol 2010;8:1037-41. [PubMed]
- Thosani N, Singh H, Kapadia A, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc 2012;75:242-53. [PubMed]
- Pouw RE, Heldoorn N, Herrero LA, et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc 2011;73:662-8. [PubMed]
- Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol 2009;104:2684-92. [PubMed]
- Inoue H, Takeshita K, Hori H, et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc 1993;39:58-62. [PubMed]
- May A, Gossner L, Behrens A, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc 2003;58:167-75. [PubMed]
- Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Safety and efficacy of multiband mucosectomy in 1060 resections in Barrett’s esophagus. Endoscopy 2011;43:177-83. [PubMed]
- Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc 2011;74:35-43. [PubMed]


