A clinical study of efficacy of polyglycolic acid sleeve after video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax
Introduction
Primary spontaneous pneumothorax (PSP) is a common disease in thoracic surgery (1). Currently, video-assisted thoracoscopic (VATS) bullectomy, followed by pleurodesis, is widely performed as the most common surgical treatment for PSP (2,3). However, prolonged postoperative air leakage is still the most frequent and troublesome early complication after the bullectomy (4). A polyglycolic acid (PGA) sleeve, which is a new absorbable reinforcement material, is confirmed to preferably reduce postoperative air leakage during lung volume reduction surgery (LVRS) (5). However, to date, its efficacy in surgeries for PSP are not fully understood. Here, we performed, to our knowledge, the first prospective study of the efficacy of PGA sleeve in preventing postoperative air leakage after VATS bullectomy for PSP.
Methods
Participants
The patients who underwent VATS bullectomy due to PSP from January 2015 to June 2016 at the Beijing Chaoyang Hospital were consecutively collected. The inclusion criteria included ipsilateral or bilateral recurrent spontaneous pneumothorax, a history of previous contralateral pneumothorax, visible blebs on the initial plain chest film or computed tomography, persistent air leakage for at least 7 days after the closed thoracic drainage, and those working in specific jobs, such as a pilot or diver. The exclusion criteria were an underlying pulmonary disease, such as chronic obstructive pulmonary disease (COPD) and bronchial asthma, a previous ipsilateral thoracic operation, pleural extensive adhesions, having a surgical contraindication, and refusal to participate in the study. The patients were randomly assigned to either the experimental group or the control group by a computer. The institutional research ethics committee of the Beijing Chaoyang Hospital approved the study (ID: 2015-S-151), and all of the patients signed the informed consent preoperatively.
Surgical approach (Figure 1)
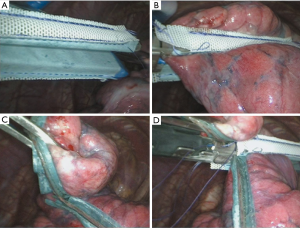
All patients underwent a 2-port unilateral VATS bullectomy under general anesthesia with a double-lumen endotracheal tube and single lung ventilation during the operation. The surgery was performed by board-certified thoracic surgeons. A 1 cm port was placed in the 7th intercostal space at the mid-axillary line for the thoracoscope, and if the patient had a closed thoracic drainage port preoperatively, then that hole was used directly. Another 3 cm working port was placed in the 4th intercostal space at the anterior axillary line. After that, the entire visceral pleural surface was examined carefully to find all of the bullae. In the experimental group, the PGA sleeve (Gunze, Ayabe, Japan) was used in combination with a 60-mm linear automatic stapler (Reach Surgical, Inc., China) in the bullectomy, while in the control group, the bullae were resected using the automatic stapler alone. After all of the bullae were resected completely, we performed a sealing test by submerging the staple lines in water and inflating the lung under 20 cm of water pressure to detect any persistent air leakage. Then, for both groups, all of the staple lines were covered with an absorbable polyglycolic acid sheet and pleural abrasion was performed. A single chest tube was placed in the thorax postoperatively, and all of the patients were reexamined with a plain chest film at the first postoperative day routinely. The patients were discharged the first day after removal of the chest tube.
Tube removal standards
We determined that there was an air leakage if bubbles continuously came out from the tube when the patient coughed several times. If no bubbles came out from the tube when the patient coughed consecutively or just a small number of bubbles came out at the beginning, we determined that there was no air leakage. On the condition that, no air leakage was detected immediately after the operation, we recorded this as air leakage day zero, and if no air leakage was detected on the first postoperative day, we recorded this as air leakage day one and so on. The chest tube was removed if there was no air leakage, there was good re-expansion of the lung as confirmed by a chest X-ray, the drainage was less than 200 mL per day, and the colour of the drainage was clear.
Follow-up
After the patients were discharged from the hospital, they all received clinical follow-up for at least 6 months. The follow-up was carried out by a clinical reexamination in our outpatient facility or by telephone.
Data collection
The clinical data included the baseline characteristics of the patients, the operative time, the number of resection margins, the number of no air leakage and air leakages lasting for more than 3 days occurred, the average postoperative air leakage, the drainage tube removal time, the postoperative hospital stay, the postoperative complications, the hospitalization expenses, and the postoperative recurrence. The baseline characteristics consisted of age, gender, body mass index (BMI), smoking habits, and the side of the pneumothorax. These data were all compared between the experimental and control groups.
Statistical analysis
The statistical analyses were conducted using IBM SPSS Statistics version 20 (SPSS Inc. Chicago, IL, USA). The continuous variables are presented as the mean ± standard deviation and were analysed by an independent sample t-test. The categorical variables are expressed as frequencies (%) and were analysed by a χ2 test. The differences were considered statistically significant when the P value was less than 0.05.
Results
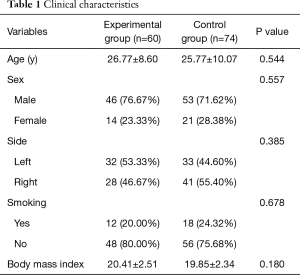
A total of 137 patients, who met the inclusion criteria, underwent VATS bullectomy from January 2015 to June 2016 at the Beijing Chaoyang Hospital. Among them, three patients, two belonging to the experimental group and one belonging to the control group, had a persistent postoperative air leakage lasting more than 10 days, therefore, these patients were removed from the study. These leakages occurred because the bases of the bullae found in these patients were wider, and thus, two or more automatic staplers were used to resect them. The staples did not overlap to achieve a seamless connection, resulting in a resection margin with a persistent air leakage. These three patients were administered an intrathoracic injection of 50% glucose and received suction, and the air leakage stopped on the 10th, 12th, and 12th postoperative day for each of the patients. Finally, 134 patients were enrolled in the study, and the experimental and the control groups consisted of 60 and 74 cases, respectively. The baseline characteristics of the patients are presented in Table 1. No statistically significant differences were observed between the two groups in terms of age, gender, BMI, smoking habits, and the side of the pneumothorax.
Full table
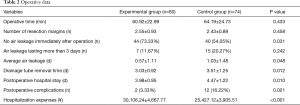
The procedures were successful for all patients with no postoperative deaths, mechanical ventilation, or serious complications. The operative data and postoperative outcomes are demonstrated in Table 2. The differences between intraoperative factors, such as the operative time (60.92±22.99 vs. 64.19±24.73 mins, P=0.433) and the number of resection margins (2.55±0.93 vs. 2.43±0.89, P=0.458) did not reach statistical significance between the two groups. We found that no air leakage was detected immediately after the operation in 44 of the 60 patients (73.33%) in the experimental group who received the PGA sleeve, and this occurred more frequently than in the control group (54.05%). While there was no significant difference in the number of air leakages lasting more than 3 days between the two groups, the average postoperative air leakage in the experimental group (0.57±1.11 days) was significantly shorter compared to the controls who did not receive the PGA sleeve (1.03±1.48 days). There was a positive correlation between the postoperative air leakage and the chest tube drainage removal time and the postoperative hospital stay, and statistically significant differences were observed for the chest tube drainage removal time (3.03±0.92 vs. 3.51±1.25 days, P=0.012) and the postoperative hospital stay (3.98±0.95 days vs. 4.47±1.22 days, P=0.010) between the two groups. While, the hospitalization expenses were clearly higher using the PGA sleeve (30,106.2±4,667.77 ¥ vs. 25,427.12±3,905.51 ¥, P<0.001). In addition, the rate of postoperative complications in the experimental group was 3.33%, and of which, one patient had a pulmonary infection and the other had postoperative atelectasis. In contrast, 12 patients (16.22%) developed complications in the control group, and this rate was significantly higher compared with the experimental group. These complications included pulmonary infection in six cases, postoperative atelectasis in three cases, and pleural effusion in three cases. All of the complications were relieved after conservative medical therapy, and none of the patients required a re-operation. The median follow-up was 16 months (range, 8 to 25 months). The mean duration of the follow-up period was (15.60±5.10 months) in the experimental group and (16.55±4.84 months) in the control group. There was no postoperative recurrence in either of the groups.
Full table
Discussion
Currently, although the spontaneous rupture of subpleural bullae is thought to be the primary cause of PSP, the reason of the formation and rupture of the bullae is not completely understood (6,7). Surgical bullectomy is the main treatment for PSP, and thus far, advances in minimally invasive techniques and perioperative care have broadened its indications. VATS bullectomy has become the standard surgical procedure for PSP, and it boasts many advantages, such as less trauma and postoperative pain, a shorter operation time, less bleeding, reduced complication rates, a shorter hospital stay, and a faster recovery (8-10).
The VATS bullectomy procedure for PSP is not complicated. However, prolonged postoperative air leakage is still the most common complication that affects the early postoperative recovery, as it can lead to pulmonary atelectasis, pleural effusion, pneumonia, or other complications, which can consequently prolong the postoperative recovery time. Moreover, keeping the thoracic drainage tube prevents postoperative cough, an important step in re-expanding the lung, which thereby increases postoperative complications even further (4). The causes of postoperative air leakage can be due to issues with the staple line of the bullectomy, the eyes of the suture, and the holes of the automatic stapler, an incomplete bullectomy, cutting of the ligature during recruitment maneuvers and damage to the lung tissue intraoperatively. Therefore, based on the reasons discussed above, various surgical techniques and additional procedures are performed to prevent air leakage, including targeting the site of the bullectomy with fibrin glue and reinforcement material. Procedures targeting the parietal pleura include mechanical parietal pleural abrasion, chemical pleural adhesions, and excision of the pleura corresponding to the pulmonary apical portion. Fibrin glue is often used in combination with covering materials, which works well for intractable air leakages. However, due to the risk of the transmission of infection pathogens, such as the human immunodeficiency virus and human hepatitis virus, it is recommended that fibrin glue be avoided (11,12). In the past decades, pleurodesis provides an excellent effect in preventing postoperative air leakage and recurrence (2,13), however, its disadvantages in diminishing pulmonary function, developing chronic chest pain and negatively affecting future thoracic surgery have made it not a routine intraoperative procedure in some countries, especially for young adults with PSP (14). A new bioabsorbable sleeve made of polyglycolic acid for staple-line reinforcement during pulmonary surgery, such as lung volume reduction surgery (LVRS), reduces postoperative air leakage well (5). However, to date, its efficacy in surgeries for PSP is unknown.
This study clearly demonstrated that using PGA sleeve in VATS bullectomy for PSP effectively prevented postoperative air leakage, especially for the early postoperative air leakage after surgery, shortened the postoperative drainage tube removal time, and reduced postoperative complications. The new reinforcement sleeve is an absorbable biosynthetic material made of PGA through a special process and does not pose the risk of disease transmission. The ductility, flexibility and histocompatibility properties of the PGA sleeve are advantageous, and it fully fuses with the surrounding tissue in the host after 3 months, causing no relevant immunity and inflammatory responses (15). Because of these excellent characteristics, the PGA sleeve, combined with an automatic stapler during surgery, can prevent air leakage. It fits closely together with the staple line of the bullectomy, occluding the holes of the automatic stapler, and reduces the risk of cutting the ligature during recruitment maneuvers. In this study, a PGA sleeve, as a reinforcement material, was combined with an automatic stapler in the experimental group, and no air leakage was detected immediately after the operation in 44 of the 60 patients (73.33%), and this was significantly higher than the control group, which did not have the sleeve, indicating that the PGA sleeve takes effect immediately after the bullectomy. Although no significant difference was observed in the number of air leakages lasting more than 3 days between the two groups, the average postoperative air leakage time in the experimental group was significantly shorter compared with the controls, which did not have a PGA sleeve (0.57±1.11 vs. 1.03±1.48 days, P=0.048). The same trend was true for the chest tube removal time and the postoperative hospital stay. A longer air leakage time requires keeping the chest tube in for more time, which could affect the postoperative cough and the residual lung re-expansion, leading to more postoperative complications. If fact, complications occurred in 12 (16.22%) of the patients in the control group, and these included pulmonary infection in six cases, postoperative atelectasis in three cases, and pleural effusion three in cases. This occurrence of complications was significantly higher than in the experimental group, which was only 3.33%. Moreover, the assembly of the PGA sleeve and the automatic stapler did not increase the operative time or the operation difficulty, as the instrument nurses can carry out this step. In addition to this, there were no sleeve-related complications associated with the operation in the experimental group, and the PGA sleeve is integrated into the host gradually. The only disadvantage was that the average hospitalization expenses correspondingly increased when the PGA sleeve was used. Previous research shows that postoperative recurrence results from the formation of new blebs near the staple line (8,16). Therefore, coverage of the visceral pleura around the staple line using an absorbable PGA sheet is useful for preventing postoperative recurrence (17,18). In this study, we covered the staple line using a PGA sheet in all patients, and no postoperative recurrence was observed in either groups during the follow-up period that ranged from 8 to 25 months. Moreover, we will perform further research to evaluate the efficacy of the PGA sleeve in preventing postoperative recurrence and the postoperative predictors of recurrence after VATS bullectomy for PSP.
The limitations of this study included the fact that it was carried out at a single institution, that a single surgeon did not perform the all of the cases, and that the follow-up period was not long enough to evaluate long-term postoperative recurrence. Therefore, further multicenter, large-scale, long-term follow-up trials are needed to compare this technique with other additional procedures for preventing prolonged postoperative air leakage and postoperative recurrence. When applying a PGA sleeve during the surgery, care should be taken to fit the sleeve together with the automatic stapler in the correct way, and one should avoid wrinkling or inverting the sleeve. In the event of that two or more automatic staplers are used to resect the bullae, the staplers must cross and overlap with each other in order to achieve a seamless connection. Otherwise, serious persistent air leakage may occur postoperatively.
This is the first prospective, randomised clinical trial of surgical methods focused on determining whether a new absorbable sleeve made of PGA can prevent postoperative air leakage after VATS bullectomy for PSP. In conclusion, using an absorbable PGA sleeve for staple line reinforcement is a simple but effective method of preventing postoperative air leakage, especially for the early postoperative air leakage after surgery, which is worthy of promotion and application in the future.
Acknowledgements
Funding: This work was supported by the Basic and Clinical Cooperation Research Fund of China Capital Medical University (15JL39).
Footnote
Conflicts of interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Research Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University (ID: 2015-S-151), and written informed consent was obtained from all patients.
References
- Noppen M, De Keukeleire T. Pneumothorax. Respiration 2008;76:121-7. [Crossref] [PubMed]
- Lang-Lazdunski L, Chapuis O, Bonnet PM, et al. Videothoracoscopic bleb excision and pleural abrasion for the treatment of primary spontaneous pneumothorax: long-term results. Ann Thorac Surg 2003;75:960-5. [Crossref] [PubMed]
- Horio H, Nomori H, Kobayashi R, et al. Impact of additional pleurodesis in video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. Surg Endosc 2002;16:630-4. [Crossref] [PubMed]
- Gomez-Caro A, Moradiellos F J, Larru E, et al. Effectiveness and complications of video-assisted surgery for primary spontaneous pneumothorax. Arch Bronconeumol 2006;42:57-61. [Crossref] [PubMed]
- Saito Y, Omiya H, Shomura Y, et al. A new bioabsorbable sleeve for staple-line reinforcement: report of a clinical experience. Surg Today 2002;32:297-9. [Crossref] [PubMed]
- Baumann MH. Management of spontaneous pneumothorax. Clin Chest Med 2006;27:369-381. [Crossref] [PubMed]
- Jantz MA, Antony VB. Pathophysiology of the pleura. Respiration 2008;75:121-33. [Crossref] [PubMed]
- Sawada S, Watanabe Y, Moriyama S. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 2005;127:2226-30. [Crossref] [PubMed]
- Freixinet JL, Canalis E, Julia G, et al. Axillary thoracotomy versus videothoracoscopy for the treatment of primary spontaneous pneumothorax. Ann Thorac Surg 2004;78:417-20. [Crossref] [PubMed]
- Naunheim KS, Mack MJ, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg 1995;109:1198-203; discussion 1203-4. [Crossref] [PubMed]
- Tokushima T, Fukuta M, Maeta H, et al. A clinical study on pulmonary fistula closure approaches using fibrin adhesives in lung surgery. Jpn J Chest Surg 2003;17:559-65. [Crossref]
- Kawamura M, Sawafuji M, Watanabe M, et al. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg 2002;73:1098-100. [Crossref] [PubMed]
- Gossot D, Galetta D, Stern JB, et al. Results of thoracoscopic pleural abrasion for primary spontaneous pneumothorax. Surg Endosc 2004;18:466-71. [Crossref] [PubMed]
- Zijl JA, Sinninghe Damste HE, et al. Video-assisted thoracoscopic introduction of talc in the treatment of recurrent spontaneous pneumothorax. Eur J Surg 2000;166:283-5. [Crossref] [PubMed]
- Minami K, Saito Y, Shomura Y, et al. A device to prevent an air-leakage after a thoracoscopic surgery for pontaneous pneumothorax. Kyobu Geka 2003;56:904-7. [PubMed]
- Sakamoto K, Kase M, Mo M, et al. Regrowth of bullae around the staple-line is one of the causes of postoperative recurrence in thoracoscopic surgery for spontaneous pneumothorax. Kyobu Geka 1999;52:939-42. [PubMed]
- Hirai K, Kawashima T, Takeuchi S, et al. Covering the staple line with a polyglycolic acid sheet after bullectomy for primary spontaneous pneumothorax prevents postoperative recurrent pneumothorax. J Thorac Dis 2015;7:1978-85. [PubMed]
- Lee S, Park SY, Bae MK, et al. Efficacy of polyglycolic acid sheet after thoracoscopic bullectomy for spontaneous pneumothorax. Ann Thorac Surg 2013;95:1919-23. [Crossref] [PubMed]


