Malignant peripheral nerve sheath tumor of the chest wall associated with neurofibromatosis: a case report
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a rare primary chest wall tumor that accounts for 5% of all soft tissue sarcomas (STSs). Any malignant tumor that arises from the peripheral nerve or from the malignant transformation of a preexisting neurofibroma is categorized as MPNST. The incidence of MPNST is 0.001% in the general population and 4.6% in patients with neurofibromatosis type 1 (NF-1, von Recklinghausen disease) (1). The tumors are highly malignant; often affect the head, trunk, and extremities; and lead to poor overall survival (2). We report a case of MPNST of the chest wall in a patient with NF-1 who developed local recurrence 5 months after R0-resection and postoperative adjuvant radiotherapy.
Case report
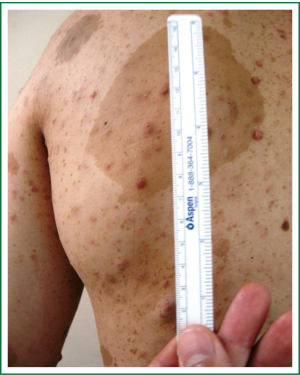
A 26-year-old male express delivery worker with a 5-pack-year history of smoking presented with a complaint of a progressively enlarging, painful, protruding mass over his left posterior chest wall since 10 months. He reported a history of NF-1 since he was a teenager. More than 6 café-au-lait spots were present on his body in addition to several skin-fold freckles on his axillary and neck base regions. Moreover, skeletal dysplasia caused a pectus carinatum appearance of his thoracic cage, and multiple neurofibromas protruding from the skin over his entire body were observed.
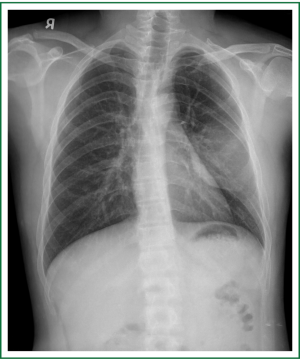
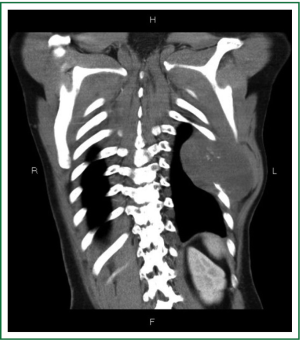
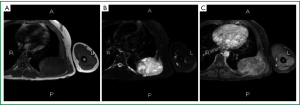
On admission, physical examination revealed a massive (approximately 7 cm × 8 cm) hard soft tissue mass bulging from his left posterior chest wall (Figure 1). Chest radiography revealed a soft tissue opacity over the left middle lung field, with the incomplete border sign that is typical for chest wall tumors (Figure 2) (3). Reconstructive computed tomography (CT) revealed a massive tumor (9.8 cm × 8.5 cm × 8.3 cm) with destruction of the left sixth and seventh ribs (Figure 3). Thoracic magnetic resonance imaging (MRI) revealed a left chest wall heterogeneous mass with low signal intensity on T1-weighted imaging (T1WI), high signal intensity on T2-weighted imaging (T2WI), and inhomogeneous contrast enhancement. The surrounding fat tissue and muscles were relatively intact (Figure 4). Malignant chest wall sarcoma was suspected and distant metastasis was ruled out by 2-deoxy-2-(18F) fluoroglucose positron emission tomography.
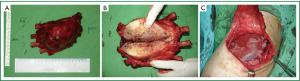
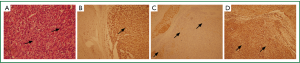
Wide excision of the lesion, resection of the fifth to eighth ribs for free surgical margins, and reconstruction with a polypropylene mesh and a latissimus dorsi muscle flap were performed (Figure 5). The patient was extubated on the second day of surgery and exhibited an uneventful recovery. Histopathologically, the sarcoma was characterized by fascicular, spindle-shaped, tumor cells with nuclear pleomorphism, frequent mitosis, and geographic necrosis. Immunohistochemical staining demonstrated an increased proliferative index (Ki-67 immunopositivity) and positive S-100 and vimentin staining, findings consistent with high-grade MPNST (Figure 6). All surgical margins were negative. Postoperative adjuvant focal radiotherapy to the chest wall was administered (60 Gy in 30 fractions over 6 weeks). Chemotherapy was not administered because a poor response and further complications were anticipated.
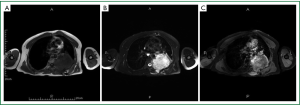
However, 5 months after surgery, the patient developed another painful protruding mass on his left chest wall near the previous surgical wound. Thoracic MRI revealed a heterogeneous lobulated mass (8.7 cm × 7.6 cm × 9.3 cm) in the left paraspinal region (posterior mediastinum) with adjacent muscle and bone involvement and extension into the spinal canal (Figure 7). Complete surgical resection could not be achieved, and salvage adjuvant chemoradiotherapy was suggested by the oncologists.
Discussion
MPNST is also referred to as malignant schwannoma, neurofibrosarcoma, and neurogenic sarcoma. These tumors are very aggressive, with an incidence of 4-10% of all STSs, and the STS staging system proposed by the American Joint Committee on Cancer is most commonly used to categorize these lesions. Two definite risk factors include NF and previous irradiation (1). More than 50% patients with MPNSTs suffer from NF-1, and the reported 5-year survival rate is only 15% (4). MPNSTs are often diagnosed in patients aged 20-50 years, do not exhibit any sex differences, and usually present as an enlarging painful mass (5).
In our patient, early diagnosis could not be made because of the slow-growing nature of the lesion and the difficulty in determining whether his back pain was myofacial or intrathoracic lesion-related. CT findings of MPNSTs usually include a large heterogeneous mass with occasional bony destruction. The signal intensity of the tumors on T1WI MRI is equal to or slightly greater than that of muscles. T2WI MRI reveals high signal intensity and heterogeneous enhancement with partial central necrosis after contrast enhancement (6). Although definite diagnosis could not be made only on the basis of imaging studies, typical MPNST imaging features were observed in our patient, which allowed differentiation from other malignant chest wall tumors and prevented preoperative biopsy.
Histopathologically, MPNSTs are characterized by atypical spindle-shaped cells with nuclear pleomorphism, frequent mitosis, and a high proliferative index. Our patient’s surgical specimen showed S-100 immunopositivity, implying malignant transformation of schwannoma (7), and vimentin immunopositivity, implying a mesenchymal origin-sarcoma (8).
The treatment of chest wall MPNSTs is multimodal, including wide local excision with minimum 4-cm safety margins, adjuvant radiotherapy, and chemotherapy (9). Local control with full oncological resection is the best indicator of good prognosis, and thoracotomy associated with rib resection or flap transfer for reconstruction may be required in these patients (10). In our patient, the MPNST was identified at the left chest wall, with sixth and seventh rib destruction and extension to the paraspinal region. We performed complete wide excision grossly, including the fifth to eighth ribs, without intraoperative frozen section analysis. However, the safety margin was quite narrow (1 mm) because of anatomic reasons (proximity to the thoracic vertebral bodies). Despite postoperative radiotherapy, local recurrence in the paraspinal region was observed 5 months after surgery.
Zou et al. (11) reported that only positive margins on microscopy were significantly associated with local recurrence of MPNSTs; age, NF-1 status, tumor site, tumor size, and addition of preoperative chemoradiotherapy were not significant factors. MPNSTs are thought to be poorly sensitive to chemotherapy and relatively radioresistant (12). Wong et al. (13) reported that adjuvant irradiation (>60 Gy) and inclusion of intraoperative electron irradiation were associated with better local disease control. Our patient received adjuvant radiotherapy because of the proximity of the surgical margin to the paraspinal region. The role of chemotherapy in MPNST treatment remains controversial. Vicent et al. (14) reported a 57% disease-free survival and an 80% overall survival 2 years after a combination of surgery, radiation, and chemotherapy using doxorubicin and ifosfamide in 10 patients with MPNSTs.
Schuetze et al. (15) reported that adjuvant chemotherapy should be considered for patients with high-grade, large, and deeply located sarcomas.
Conclusions
In conclusion, although primary tumors of the chest wall are relatively rare, MPNSTs should be included in the differential diagnoses of chest wall tumors in patients with NF-1. For select patients with high-risk soft tissue sarcoma, intraoperative frozen section analysis can be used for confirmation of adequate surgical margins; furthermore, administration of routine adjuvant chemotherapy should be considered after complete resection in these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986;57:2006-21. [PubMed]
- Tucker T, Wolkenstein P, Revuz J, et al. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 2005;65:205-11. [PubMed]
- Schiffman SR, Datta V, Wandtke J, et al. Imaging features of chest wall tumors. Contemp diagn radiol 2012;35:1-5.
- Leroy K, Dumas V, Martin-Garcia N, et al. Malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1: a clinicopathologic and molecular study of 17 patients. Arch Dermatol 2001;137:908-13. [PubMed]
- Richards JB, Marotti J, DeCamp MM, et al. Thoracic malignant peripheral nerve sheath tumor: a case report and literature review. Clin Pulm Med 2012;19:298-301.
- Tateishi U, Gladish GW, Kusumoto M, et al. Chest wall tumors: radiologic findings and pathologic correlation: part 2. Malignant tumors. Radiographics 2003;23:1491-508. [PubMed]
- Gnanalingham K, Bhattacharjee S, ONeill K. Intraosseous malignant peripheral nerve sheath tumor (MPNST) of the thoracic spine: a rare cause of spinal cord compression. Spine (Phila Pa 1976) 2004;29:E402-5.
- Lee CF, Luo JW, Liu CJ. Undifferentiated sarcoma of the mandible: a case report. Taiwan J Oral Surg 2009;20:263-71.
- David EA, Marshall MB. Review of chest wall tumors: a diagnostic, therapeutic, and reconstructive challenge. Semin Plast Surg 2011;25:16-24. [PubMed]
- Tsukushi S, Nishida Y, Sugiura H, et al. Soft tissue sarcomas of the chest wall. J Thorac Oncol 2009;4:834-7. [PubMed]
- Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg 2009;249:1014-22. [PubMed]
- Cheng SF, Chen YI, Chang CY, et al. Malignant peripheral nerve sheath tumor of the orbit: malignant transformation from neurofibroma without neurofibromatosis. Ophthal Plast Reconstr Surg 2008;24:413-5. [PubMed]
- Wong WW, Hirose T, Scheithauer BW, et al. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys 1998;42:351-60. [PubMed]
- Moretti VM, Crawford EA, Staddon AP, et al. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol 2011;34:417-21. [PubMed]
- Schuetze SM, Patel S. Should patients with high-risk soft tissue sarcoma receive adjuvant chemotherapy? Oncologist 2009;14:1003-12. [PubMed]