A preliminary exploration of the intravoxel incoherent motion applied in the preoperative evaluation of mediastinal lymph node metastasis of lung cancer
Introduction
Lung cancer is one of the most common malignant tumours in the world and the No. 1 cause of death among malignant tumours. According to statistics, in 2012 alone, there were approximately 1.8 million cases of newly diagnosed lung cancer, accounting for 12.9% of all malignant tumours, and approximately 1.59 million people (19.4%) died of lung cancer (1).
In the past, the preoperative N staging of lung cancer mainly depended on a chest computed tomography (CT) scan; this approach is based on morphology and measurement of the diameter only, so the sensitivity and specificity are usually low. With the application of positron emission tomography (PET)/CT, the accuracy of preoperative staging of lung cancer has improved (2), but because the inflammatory reactive hyperplasia of mediastinal lymph nodes caused by tuberculosis also manifests as enhanced metabolism, the accuracy of PET/CT is low, and its false positive rate is relatively high (3,4). Therefore, rapid, non-invasive and accurate N staging for patients with lung cancer remains a challenge.
In recent years, functional magnetic resonance imaging (MRI) technology has undergone revolutionary development, leading to a wide range of applications. Compared with CT and PET, MRI has no radiation exposure, involves no contrast agents, can obtain many imaging parameters and sequences, and can provide much more abundant information for physicians than CT imaging, which relies on a single X-ray attenuation coefficient (5).
Diffusion weighted imaging (DWI) is an indirect measurement of the microstructure of the tissue that detects the direction and extent of the diffusion of water molecules in the tissue. Intravoxel incoherent motion (IVIM) imaging is a new technology first proposed by Le Bihan et al. in 1986 (6) and developed based on DWI. IVIM not only provides the parameters of water molecule movement in the tissue but also reflects the degree of tissue perfusion. IVIM is still in the research stage, with most studies on head and neck tumours and some related research on liver, kidney and breast lesions (7-9), but its application in the diagnosis and treatment of lung and mediastinal diseases has rarely been reported (10,11). Therefore, the aim of this study was to evaluate the applicability of this technique in the preoperative evaluation of mediastinal lymph node metastasis in lung cancer by comparing the differences in the IVIM sequence parameters between metastatic and non-metastatic lymph nodes in lung cancer.
Methods
Inclusion criteria
Patients with the following were included: (I) complete preoperative examination data and postoperative pathological results; (II) a primary lung mass, with no previous history of other malignancies; (III) no preoperative radiotherapy, chemotherapy, targeted therapy, immunization therapy or other form of anti-tumour therapy; (IV) overall good general condition, with no severe medical co-morbidities, no distant metastases or other related surgical contraindications, and a candidate for surgical treatment; and (V) underwent lobectomy and standard mediastinal lymph node dissection as the surgical procedure.
Exclusion criteria
Patient with the following were excluded: (I) MRI examination could not be performed due to relevant contraindications; (II) preoperative radiotherapy, chemotherapy, targeted therapy, immunotherapy or other forms of anti-tumour therapy; (III) severe medical co-morbidities or advanced cancer revealed on the preoperative examination, so surgery could not be performed; (IV) postoperative pathology showed malignant tumour metastasis or benign disease in other systems; or (V) refusal of MRI examination or surgery.
Patient data collection
From October 2015 to June 2016, 66 patients with lung cancer were admitted, of whom 50 underwent thoracic enhanced CT in our hospital and 16 had undergone chest CT at other hospitals prior to admission. This study had the consent of the patients themselves and their families, with a signed MRI informed consent.
This study included 31 males and 35 females, ages 35–77 years old, with a mean age of 59.68±10.06 years.
According to the 2015 lung cancer classification by the WHO, there were 12 cases of squamous cell carcinoma, 48 cases of adenocarcinoma, 1 case of adenosquamous carcinoma, 3 cases of neuroendocrine tumour (1 case of small cell lung cancer and 2 cases of atypical carcinoid), 1 case of large cell carcinoma, and 1 case of sclerosing lung tumour.
The MRI and CT results were reviewed by two senior imaging experts and a thoracic surgeon who individually rendered a corresponding diagnosis based on the imaging characteristics and parameters.
According to the MRI results of the 50 patients with complete examination data, the IVIM parameters [apparent diffusion coefficient (ADC), diffusion coefficient (D), pseudo-diffusion coefficient (D*) and perfusion fraction (f)] and the short axis diameter of the lymph nodes were measured. Because N1 lymph nodes and lymph nodes with a diameter <4 mm were difficult to display in the scanning images, the phase parameters could not be measured, so the data for those cases were discarded. Furthermore, because CT imaging results of groups 8 and 9 lymph nodes were rare, they were also excluded from the statistics (12).
Statistical analysis
Fisher’s exact test was performed for the MRI, CT and pathological results. The ADC, D, D*, and f parameters and the short axis diameters of the benign and malignant lymph nodes in 50 patients were statistically analysed using the Mann-Whitney U test, and a difference with P<0.05 was considered statistically significant. The ROC was plotted, and the area under the curve (AUC) was calculated to find the optimal diagnostic cut-off. The parameter data of the remaining 16 patients were used for validation. In this study, all of the statistical results are represented as the mean ± standard deviation, and all of the statistical analysis and charts were prepared using IBM SPSS 18.0 statistical software or GraphPad Prism 6.01 software.
Results
In this study, a total of 66 patients underwent dissection of 273 groups of lymph nodes, and metastases were identified on postoperative pathological analysis in 20 groups (Table 1).

Full table
A mediastinal lymph node showing a short axis diameter >10 mm on CT was considered a metastasis (13). Fisher’s exact test was performed for the CT, MRI, and pathological results, and P<0.05 was considered statistically significant (Table 2). The MRI and pathological results were further analysed with the Kappa consistency test, and the results showed a Kappa value of 0.732, indicating satisfactory consistency, with P<0.001, indicating that the difference was significant. Thus, the diagnostic accuracy of IVIM examination is good.

Full table
According to the pathological results, the IVM parameters (ADC, D, D*, f) and short axis diameter of the metastatic and non-metastatic lymph nodes were compared using the Mann-Whitney U test (Table 3). The average ADC and D values of the metastatic lymph nodes were significantly lower than for the non-metastatic lymph nodes, showing statistically significant differences with P<0.01. Thus, these two parameters can be used for the differential diagnosis. There were no statistically significant differences in the parameters D*, f or short axis diameter between the metastatic and non-metastatic lymph nodes, so these three parameters cannot be used to identify lymph node metastases. The ROC curves were plotted against the IVIM parameters of the lymph nodes (Figures 1,2), and the respective AUCs were calculated (Table 4). The AUC of the ADC was 0.871, with P<0.01, the optimal diagnostic cut-off was 1.890×10−3 mm2/s, the diagnostic sensitivity was 92.7%, the specificity was 80.0%, and the Youden index was 0.727. The AUC of D was 0.740, with P<0.01, the optimal diagnostic cut-off was 0.648×10−3 mm2/s, the diagnostic sensitivity was 70.0%, the specificity was 84.1%, and the Youden index was 0.541.

Full table
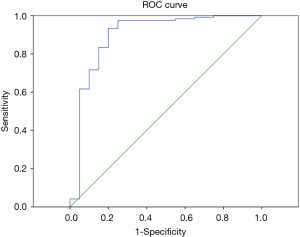
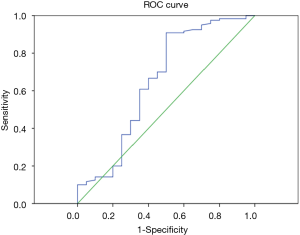

Full table
The remaining 16 patients underwent dissection of 57 groups of lymph nodes. The pathological results showed that 12 groups were metastatic lymph nodes and 45 groups were non-metastatic lymph nodes. The optimal diagnostic cut-offs based on the ADC and D data were used as the diagnostic criteria (benign ADC >1.890×10−3 mm2/s, benign D >0.344×10−3 mm2/s; malignant ADC <1.890×10−3 mm2/s, malignant D <0.344×10−3 mm2/s). According to the Kappa consistency test, the consistency using ADC as the diagnostic criterion was not significantly different; the consistency using D as the diagnostic criterion was slightly worse; the consistency was significantly increased using the combination of ADC and D (Table 5).
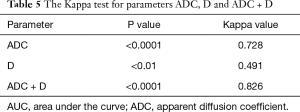
Full table
Discussion
As one of the latest MRI technologies, IVIM is more and more widely applied in the study of various human organs. In recent years, some studies have used IVIM to identify benign and malignant tumours (14,15), and the peritoneal lymph node metastasis of rectal cancer (16,17). However, the application of IVIM in the diagnosis of mediastinal lymph node metastasis of lung cancer has not been reported.
IVIM theory assumes that blood microcirculation is non-uniform and unordered random movement and that displacement of blood may occur by magnetization, resulting in signal attenuation. The ADC can be integrated by the nonlinear least squares method, which includes both diffusion and perfusion. IVIM assumes that the microscopic movement of organisms is divided into two types. One is slow movement, with diffusion according to Brownian motion, and it describes the random distribution of the blood in the circulatory network from the macroscopic perspective. The pseudo diffusion coefficient (D*) describes this perfusion. The other type of movement is fast movement, which is affected by the pressure gradient and is described with the diffusion coefficient (D), reflecting the extravascular spin and intercellular fluid movement and describing diffusion (16). Therefore, IVIM is an advanced non-invasive imaging technology with the simultaneous detection of the diffusion of water molecules and capillary perfusion within tissues. Through image processing, the values of the parameters f, D* and D can be obtained, which represent the ratio of the voxel capillary volume to the total tissue volume, the microcirculation perfusion of the capillary network, and the diffusion state of the water molecules in the tissue, respectively (17,18). No external contrast agent is needed. This detection method can allow the corresponding diagnosis at the molecular level before the occurrence of morphological changes in the tissue, thus truly achieving early diagnosis and early treatment. It has great potential for diagnosis, with prospects for wide clinical applications.
The ADC value reflects diffusion capability. Studies have shown that cell density is the main factor influencing the dispersion of water molecules in tumours (4). The cell density in metastatic lymph node tissue is high, and water dispersion is significantly limited, so the ADC value is low; in contrast, the cell density of non-tumour cells is relatively low, and the ADC value is high. In this study, the mean ADC of metastatic lymph nodes was significantly lower than the mean ADC of the non-metastatic lymph nodes. This result is in line with theory. The findings of Liu et al. on the lymph node metastasis of rectal cancer were also consistent with the findings in the present study.
D is the diffusion coefficient, reflecting the diffusion of water molecules in the tissue, which mainly depends on the cell density and the components of the extracellular matrix. Studies have shown that the D value is negatively correlated with the cell structure and the ratio of the nucleus to the cytoplasm, that is, the higher the number of cells and the higher the ratio of the nucleus to the cytoplasm, the smaller the extracellular space is, so the diffusion movement of the water molecules is limited, and the D value is decreased (19). In this study, the mean D value of the metastatic lymph nodes was significantly lower than the D value of the non-metastatic lymph nodes; this result is in line with theory. The findings of Qiu et al. and Yu et al. are also similar to the results of this study. In this study, the combination of the parameters ADC and D as the diagnostic criteria could further improve the diagnostic efficiency.
The pseudo-diffusion coefficient, D*, is proportional to the blood flow rate and the mean length of the capillaries and is associated with the perfusion of tumour vessels and the microcirculation in metastatic lymph nodes (20). In this study, the D* of the metastatic lymph nodes was slightly lower than the D* of the non-metastatic lymph nodes, but the difference was minor and not significant. This result is different from the results of Qiu et al. and Yu et al. In those two studies on rectal cancer, the D* values in metastatic lymph nodes and non-metastatic lymph nodes were statistically significantly different, and the mean D* value of the metastatic lymph nodes was significantly lower than the mean D* value of the non-metastatic lymph nodes. Similar findings have been reported in studies on certain head and neck malignancies (21). However, in other studies on axillary lymph node metastasis of breast cancer, the results are the opposite (22,23). These opposite findings may be caused by the different parts and different types of primary tumours.
In addition to D*, f is also an important parameter in the IVIM sequence that reflects perfusion, with the capillary blood flow in each voxel. In other words, f can reflect the proportion of water molecules in the capillary network in the MRI scanning range. Therefore, f is positively correlated with tissue perfusion. In this study, the f value of the metastatic lymph nodes was slightly higher than the f value of the non-metastatic lymph nodes, but the difference was not significant. In the study by Yu et al., the f value of the non-metastatic lymph nodes was higher than the f value of the metastatic lymph nodes, with no statistically significant difference (P=0.051). However, other studies showed that the f value of metastatic lymph nodes was higher than the f value of non-metastatic lymph nodes and that the f value of benign lesions was higher than the f value of malignant lesions, including for nasopharyngeal cancer vs. adenoid hypertrophy (24), lung malignancy vs. obstructive pulmonary disease (25), and prostate cancer vs. benign prostatic hyperplasia and prostatitis (26). One possible reason for these different results may be that different types of lesions may greatly affect f value results. At the same time, other factors, including the characteristics of the tissues in the magnetic field, the echo time, and the relaxation time of the MRI scanning, may also greatly affect the f value (27,28).
Traditional enhanced CT can determine lymph node metastases mainly by measuring the short axis diameter of lymph nodes. If tumour metastasis to lymph nodes occurs, hyperplasia will cause enlargement of the lymph nodes. In general, if the short axis diameter of a lymph node is >1 cm, then metastasis in this lymph node is suspected. In this study, the comparison of the diagnostic efficiency of CT, MRI and pathological results for lymph node metastasis showed that the sensitivity, specificity and accuracy of MR imaging in diagnosing mediastinal lymph node metastasis were superior to enhanced CT. One possible reason may be that lung cancer screening is now relatively common, so the disease can be found at an early stage with no lymph node metastasis, and most lymph nodes revealed by CT are caused by infectious inflammation rather than tumour metastasis. In our study, the average short axis diameter of metastatic lymph nodes was slightly higher than that of non-metastatic lymph nodes, but the difference was not statistically significant (P=0.131), showing no special diagnostic efficiency. This result shows that the short axis diameter of the lymph nodes is not significantly related to metastasis when the lymph nodes are small. Studies have shown that the possibility of metastasis is close to 15–42% in lymph nodes with a diameter <5 mm (29). In other words, in cases of lung cancer with mediastinal lymph node metastasis but the lymph node enlargement is not yet apparent, traditional CT is unable to accurately stage such cases. However, IVIM can provide the appropriate diagnosis by measuring the corresponding parameters to reflect the changes at the molecular level before the macro-level changes occur.
In this study, the PET/CT was performed in few patients and none of the patients received EBUS-TBNA. So it was impossible to compare the accuracy of IVIM with PET/CT or EBUS-TBNA. But there were some studies have shown that use the FDG-PET assess the mediastinal lymph nodes in NSCLC has a sensitivity of 74% (69–79%) and a specificity of 85% (82–88%). FDG-PET has a poor sensitivity for small lymph nodes and a poor specificity for large lymph nodes (20% false positives) (30). Another shortcoming of FDG-PET is radioactive and costly. EBUS-TBNA had a sensitivity of 93% (91–94%) and a specificity of 100% (99–100%). EBUS-TBNA is effective for sampling small lymph nodes (ranging from 5 to 9 mm) (31), its minimally invasive, safe, well tolerated, and rarely need general anaesthesia. However, not all lymph node regions can be sampled by transbronchial biopsy. Therefore, further study need to be performed to compare the accuracy of IVIM, PET/CT and EBUS-TBNA using in the diagnosis of the mediastinal lymph nodes metastasis preoperatively.
Of course, this study still has some limitations that may affect the experimental results. First, the total sample size was small. Second, the N1 group and the lymph nodes with a short axis diameter <4 mm were unclear in the images. Therefore, in this study, the data for the N1 lymph nodes and the lymph nodes with a short axis diameter <4 mm were discarded. As described earlier, in rectal malignancies, the possibility of metastasis to the lymph nodes with a diameter <5 mm is close to 15−42% (29); therefore, the discarded data on these lymph nodes might lead to selection bias, thus affecting the conclusion.
Conclusions
The results of this study preliminarily showed that certain IVIM parameters, such as the ADC and diffusion coefficient (D), were significantly different between lung cancer patients with and without metastatic lymph nodes. The differential diagnosis of mediastinal lymph node metastasis can be achieved based on these parameters. Therefore, IVIM-DWI can be used to preoperatively determine mediastinal lymph node metastasis of lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional research ethics committee of Beijing Chao-Yang Hospital (2014-S-166) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000;343:254-61. [Crossref] [PubMed]
- Usuda K, Zhao XT, Sagawa M, et al. Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Ann Thorac Surg 2011;91:1689-95. [Crossref] [PubMed]
- Nomori H, Mori T, Ikeda K, et al. Diffusion-weighted magnetic resonance imaging can be used in place of positron emission tomography for N staging of non-small cell lung cancer with fewer false-positive results. J Thorac Cardiovasc Surg 2008;135:816-22. [Crossref] [PubMed]
- Cieszanowski A, Lisowska A, Dabrowska M, et al. MR imaging of pulmonary nodules: detection rate and accuracy of size estimation in comparison to computed tomography. PLoS One 2016;11:e0156272. [Crossref] [PubMed]
- Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401-07. [Crossref] [PubMed]
- Lemke A, Laun FB, Simon D, et al. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med 2010;64:1580-5. [Crossref] [PubMed]
- Chandarana H, Kang SK, Wong S, et al. Diffusion-weighted intravoxel incoherent motion imaging of renal tumors with histopathologic correlation. Invest Radiol 2012;47:688-96. [Crossref] [PubMed]
- Bokacheva L, Kaplan JB, Giri DD, et al. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J Magn Reson Imaging 2014;40:813-23. [Crossref] [PubMed]
- Uto T, Takehara Y, Nakamura Y, et al. Higher sensitivity and specificity for diffusion-weighted imaging of malignant lung lesions without apparent diffusion coefficient quantification. Radiology 2009;252:247-54. [Crossref] [PubMed]
- Ohno Y, Hatabu H, Takenaka D, et al. Metastases in mediastinal and hilar lymph nodes in patients with non-small cell lung cancer: quantitative and qualitative assessment with STIR turbo spin-echo MR imaging. Radiology 2004;231:872-79. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Kamiyoshihara M, Kawashima O, Ishikawa S, et al. Mediastinal lymph node evaluation by computed tomographic scan in lung cancer. J Cardiovasc Surg (Torino) 2001;42:119-24. [PubMed]
- Sumi M, Nakamura T. Head and neck tumors: assessment of perfusion-related parameters and diffusion coefficients based on the intravoxel incoherent motion model. AJNR Am J Neuroradiol 2013;34:410-6. [Crossref] [PubMed]
- Du J, Li K, Zhang W, et al. Intravoxel incoherent motion MR imaging: comparison of diffusion and perfusion characteristics for differential diagnosis of soft tissue tumors. Medicine 2015;94:e1028. [Crossref] [PubMed]
- Qiu L, Liu XL, Liu SR, et al. Role of quantitative intravoxel incoherent motion parameters in the preoperative diagnosis of nodal metastasis in patients with rectal carcinoma. J Magn Reson Imaging 2016;44:1031-9. [Crossref] [PubMed]
- Yu XP, Wen L, Hou J, et al. Discrimination between metastatic and nonmetastatic mesorectal lymph nodes in rectal cancer using intravoxel incoherent motion diffusion-weighted magnetic resonance imaging. Acad Radiol 2016;23:479-85. [Crossref] [PubMed]
- Kim T, Kim SG. Quantification of cerebral arterial blood volume using arterial spin labeling with intravoxel incoherent motion-sensitive gradients. Magn Reson Med 2006;55:1047-57. [Crossref] [PubMed]
- Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497-505. [Crossref] [PubMed]
- Razek AA, Fathy A, Gawad TA. Correlation of apparent diffusion coefficient value with prognostic parameters of lung cancer. J Comput Assist Tomogr 2011;35:248-52. [Crossref] [PubMed]
- Gloria C, Li Q, Xu L, et al. Differentiation of diffusion coefficients to distinguish malignant and benign tumor. J Xray Sci Technol 2010;18:235-49. [PubMed]
- Le Bihan D, Turner R. The capillary network: a link between IVIM and classical perfusion. Magn Reson Med 1992;27:171-78. [Crossref] [PubMed]
- Fischbein NJ, Noworolski SM, Henry RG, et al. Assessment of metastatic cervical adenopathy using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol 2003;24:301-11. [PubMed]
- Kvistad KA, Rydland J, Smethurst HB, et al. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol 2000;10:1464-71. [Crossref] [PubMed]
- Murray AD, Staff RT, Redpath TW, et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. Br J Radiol 2002;75:220-28. [Crossref] [PubMed]
- Zhang SX, Jia QJ, Zhang ZP, et al. Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol 2014;24:1998-2004. [Crossref] [PubMed]
- Wang LL, Lin J, Liu K, et al. Intravoxel incoherent motion diffusion-weighted MR imaging in differentiation of lung cancer from obstructive lung consolidation: comparison and correlation with pharmacokinetic analysis from dynamic contrast-enhanced MR imaging. Eur Radiol 2014;24:1914-22. [Crossref] [PubMed]
- Liu X, Peng W, Zhou L, et al. Biexponential apparent diffusion coefficients values in the prostate: comparison among normal tissue, prostate cancer, benign prostatic hyperplasia and prostatitis. Korean J Radiol 2013;14:222-32. [Crossref] [PubMed]
- Sumi M, Van Cauteren M, Sumi T, et al. Salivary gland tumors: use of intravoxel incoherent motion MR imaging for assessment of diffusion and perfusion for the differentiation of benign from malignant tumors. Radiology 2012;263:770-77. [Crossref] [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edn). Chest 2007;132:178S-201S.
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]


