Systolic anterior motion of the mitral valve—the mechanism of postural hypotension following left intrapericardial pneumonectomy
Introduction
Systolic anterior motion (SAM) is defined as displacement of the distal portion of the anterior leaflet of the mitral valve toward the left ventricular outflow area (LVOA) (1). SAM can occur in patients without hypertrophic cardiomyopathy (HOCM) (2) and is a well-recognized cause for unexplained sudden hypotension in perioperative settings (3). We present a case of persistent orthostatic hypotension caused by SAM following left intrapericardial pneumonectomy and mediastinal lymph node dissection for squamous cell carcinoma of the lung invading intrapericardial portion of the inferior pulmonary vein.
Case presentation
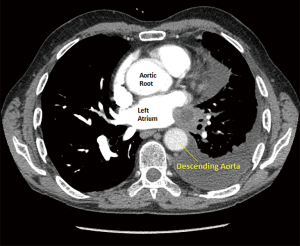
A 67-year-old male presented with squamous cell carcinoma arising in the left lower lobe and invading the inferior pulmonary vein into the left atrium (Figure 1). Preoperative invasive endobronchial ultrasound staging demonstrated N0 status, and there was no evidence of distant metastases. Due to the partial invasion of the left atrium (T4), patient underwent four cycles of neoadjuvant platinum based doublet chemotherapy with excellent clinical response (we could show figure here). Since the left lung contributed only 12% of overall lung function, surgical plan was to perform left intrapericardial pneumonectomy, via left thoracotomy.
The operation began with mediastinoscopy, which showed no evidence of nodal disease. Subsequent left thoracotomy was performed and intercostal muscle harvest for the reinforcement of the bronchus. Pericardium was then opened to allow for the dissection of the inferior pulmonary vein. The confluence of the inferior and superior vein was densely adhered and could not be safely separated. Decision was made to continue with pneumonectomy. The left main pulmonary artery was identified and stapled with a vascular staple load, the left mainstem bronchus was than dissected to the level of carina and divided with a stapler. Both inferior and superior pulmonary veins were subsequently divided en bloc with partial atrial resection utilizing 60 mm TA stapler with blue (3.5 mm) staple load. Essentially all pericardium to the left of the midline was also resected en bloc with the specimen. Since the heart was laying anatomically oriented in the left chest without any tension on the venous return, and because of the very large size of pericardial defect, the pericardium was not reconstructed. Chest cavity was closed with one drain in place connected to post pneumonectomy pleurovac.
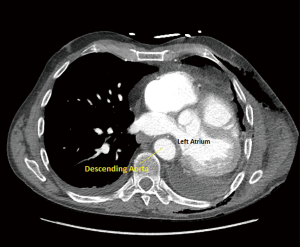
Patient remained hemodynamically stable for the first 24 hours, however, on postoperative day (POD) 2 started experiencing severe postural hypotension {81/53 [62] mmHg}, which temporarily responded to fluid boluses, and subsequently required phenylephrine infusion at 50 mcg per minute. Patient also manifested hepatojugular reflux with jugular venous distention. Right heart failure and pulmonary hypertension were considered in the differential diagnosis, and bedside transesophageal echocardiography (TEE) showed the right ventricle mildly dilated with mildly depressed right ventricular systolic function, dilation of the right atrium, and left atrium small in size. Computed tomography (CT) scan of the chest, however, demonstrated evidence of left atrial compression, suggesting dynamic obstruction of the venous return from the remaining right-sided pulmonary veins (Figure 2). Due to the patient’s hemodynamic instability off of phenylephrine, the decision was made to take the patient back to the operating room for a redo thoracotomy and a pericardial reconstruction with bovine pericardium (12×25 cm bovine pericardium) on POD 4.
On arrival to the operating room, patient was in the supine position and demonstrated stable hemodynamics with four liters of supplemental oxygen and phenylephrine infusion at 0.3 mcg/kg/min. A pre-induction arterial line was placed and vital signs were as follows; blood pressure 139/72 [99] mmHg, heart rate of 84 bpm, respiratory rate of 14, and oxygen saturation of 95%. After a smooth induction the patient was easily intubated with a single lumen endotracheal tube. A 9-French central line was placed in the right internal jugular vein with ultrasound guidance. Upon insertion of the central line, the patient’s baseline CVP was 23 mmHg in supine position. An orogastric tube was inserted to empty and decompress the stomach and was removed easily for insertion of a TEE.
On TEE a mid-esophageal four-chamber view was obtained and revealed a left atrial outflow obstruction (Figure 3). This was due to the heart being flipped over the aorta and hence compressing the outflow tract of the atrium above the mitral annulus. Given the abnormal orientation of the heart, the geometry of the mitral annulus was altered as well. In addition, the left ventricle was under filled given the left atrial outflow tract obstruction; this under filling was also confirmed on a short axis trans-gastric view. Further examination via a mid-esophageal aortic valve long axis view showed the presence of SAM of the anterior leaflet of the mitral valve causing LVOA obstruction.
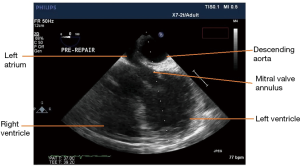
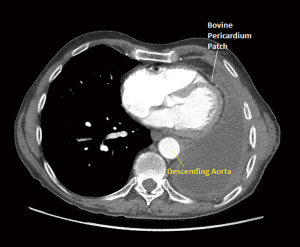
Patient was placed in right lateral decubitus position and once TEE examination was performed the above anatomic changes resolved. The left atrial outflow tract was no longer obstructed by the aorta. The left ventricular filling was normalized and no further evidence of SAM was seen through the examination. The pericardial reconstruction was then performed. Upon identification of the edges of the pericardium, a 12×25 cm patch of bovine pericardium was used and sutured (3-0 Prolene) to the rim of the patient’s pericardium to create a sling for the heart to avoid cardiac distortion and compression by the descending thoracic aorta. At the end, the reconstruction was under appropriate tension and when the patient was positioned back in supine position his hemodynamics were maintained without any need for vasopressors.
On post-operative TEE, the aorta was no longer obstructing the left atrium, the left ventricle was properly filled, and there was no evidence of SAM. The patient’s hemodynamics was maintained and there was no further need for pressor support. Emergence from the anesthetic was unremarkable and the patient was extubated and taken to the post anesthesia care unit (PACU) in stable condition. The postoperative repeat echocardiogram showed resolution of previous findings and he was able to stay off vasopressors post-surgery. On POD 4 following reoperation the patient was discharged home in a stable condition (Figure 4).
Discussion
Hypotension is a very common perioperative complication seen in patients under general or regional anesthesia. The most common etiologies attributing to perioperative hypotension are venodilation and hypovolemia due to delivered anesthetics, acute cardiac failure, systemic inflammation, sepsis, anaphylaxis, arrhythmias, tension pneumothorax, or pericardial tamponade (4,5). An etiology that is recognized in cardiac literature, but is uncommon in non-cardiac surgery is perioperative dynamic left ventricular outflow obstruction due to SAM on the mitral valve (6). In cardiac patients this condition is seen in patients with HOCM, apical ballooning syndrome, or following mitral valve surgery in which the geometry of the annulus has been changed and the annular conformation leads to SAM (7). Surgical or percutaneous aortic valve replacement may represent a cause of post-operative SAM and hypotension, albeit 1.6% (8). There are case reports in the literature describing acute perioperative hypotension in non-cardiac surgery, these include laparoscopic surgery, hepatobiliary surgery, orthopedic surgery, and major abdominal procedures (2,6,9-11). In addition, it has also been reported that the development of SAM during dobutamine stress echocardiography is not uncommon in patients without known HOCM, and patients who develop SAM during dobutamine infusion exhibit predisposing echocardiographic abnormalities (12). Initial management strategy of the patient with SAM diagnosis includes treatment with beta-adrenoceptor blockade, volume loading, and increase in afterload to correct SAM in the vast majority of patients.
In this case we are presenting perioperative hypotension following intrapericardial pneumonectomy, which was precipitated by the absence of pericardial reconstruction and patient’s positioning in the supine position in the post-operative period. Given the challenges of managing patient’s hypotension he was brought to the operating room where on TEE examination it was apparent that there was LVOA obstruction due to SAM of the mitral valve. This was precipitated by geometrical conformational changes to the heart, which was flipped over the aorta. This caused left atrial outflow obstruction, which led to absolute reductions in the left ventricular preload, which in turn caused the SAM of the mitral valve and the resulting hypotension. When the geometric conformational changes were repaired patients heart anatomy was normalized and hypotensive events were instantly corrected.
Hypotension and low cardiac output syndrome, both consequences of SAM, do not respond to the typical treatment guidelines, thus if the presence of LVOA obstruction and SAM is confirmed, a correct fluid therapy, negative inotropes and vasoconstrictors should be implemented (13). As previous reported cases, fluid management and a correct identification of the underlying SAM etiology, brought the resolution of symptoms and disappearance of the LVOA obstruction (9). Echocardiography with evaluation of LVOA in terms of obstruction is crucial to the diagnosis of SAM (13). Transthoracic echocardiogram (TTE) can be used as a first line diagnostic tool (10), however, TEE is recommended if TTE is limited, especially in hemodynamically unstable patients (3).
Conclusions
We report a case of a patient who developed persistent postural hypotension following left intrapericardial pneumonectomy. The correct evaluation with intraoperative TEE visualization was able to determine the underlying cause of SAM and following surgical repair able to confirm resolution. In light of the above reported case, the pericardium should be preferentially reconstructed after left sided intrapericardial pneumonectomy to preserve normal cardiac anatomy and function after complex interventions, and clinicians should be aware that SAM can be an underlying cause of hypotension even in patients without HOCM.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery. Chapter 15. Maryland Heights: Saunders Elsevier, 2010.
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287:1308-20. [Crossref] [PubMed]
- Fujita Y, Kagiyama N, Sakuta Y, et al. Sudden hypoxemia after uneventful laparoscopic cholecystectomy: another form of SAM presentation. BMC Anesthesiol 2015;15:51. [Crossref] [PubMed]
- Griffiths C, Agarwal R. Hypotension. In: Duke J, Rosenbert SG, eds. Anesthesia secrets, 2nd ed. Philadelphia: Hanley & Belfus Inc., 2000:157-61.
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001;345:588-95. [Crossref] [PubMed]
- Luckner G, Margreiter J, Jochberger S, et al. Systolic anterior motion of the mitral valve with left ventricular outflow tract obstruction: three cases of acute perioperative hypotension in noncardiac surgery. Anesth Analg 2005;100:1594-8. [Crossref] [PubMed]
- Hymel BJ, Townsley MM. Echocardiographic assessment of systolic anterior motion of the mitral valve. Anesth Analg 2014;118:1197-201. [Crossref] [PubMed]
- Grasso C, Scandura S, Buccheri S, et al. MitraClip Implantation for the Treatment of New-Onset Systolic Anterior Motion of the Mitral Valve After Transcatheter Aortic Valve Replacement. Ann Thorac Surg 2016;102:e517-9. [Crossref] [PubMed]
- Cavallaro F, Marano C, Sandroni C, et al. Systolic anterior motion causing hemodynamic instability and pulmonary edema during bleeding. Minerva Anestesiol 2010;76:653-6. [PubMed]
- Chockalingam A, Dorairajan S, Bhalla M, et al. Unexplained hypotension: the spectrum of dynamic left ventricular outflow tract obstruction in critical care settings. Crit Care Med 2009;37:729-34. [Crossref] [PubMed]
- Reddy S, Ueda K. Unexpected refractory intra-operative hypotension during non-cardiac surgery: Diagnosis and management guided by trans-oesophageal echocardiography. Indian J Anaesth 2014;58:51-4. [Crossref] [PubMed]
- Meimoun P, Benali T, Sayah S, et al. Significance of systolic anterior motion of the mitral valve during dobutamine stress echocardiography. J Am Soc Echocardiogr 2005;18:49-56. [Crossref] [PubMed]
- Sobczyk D. Dynamic left ventricular outflow tract obstruction: underestimated cause of hypotension and hemodynamic instability. J Ultrason 2014;14:421-7. [Crossref] [PubMed]