Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum
Introduction
Esophageal cancer is the eight most common cancer worldwide and the sixth most common cause of death from cancer (1) .The cornerstone of curative treatment for esophageal cancer is neoadjuvant chemoradiotherapy followed by esophagectomy with a locoregional lymphadenectomy. Surgery combined with neoadjuvant chemo-radiotherapy results in a 5-year overall survival of 47% (2-5).
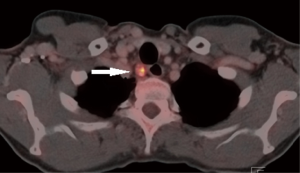
An exception are patients with esophageal cancer extending into the upper mediastinum or neck, those with a tumor involving the upper 1/3 part of the esophagus or paratracheal pathologic lymph nodes at level 2 and/or 4 (Figure 1). Because of limitations in surgical techniques (e.g., confined working space and poor overview) these patients are often precluded from surgery and treated with definite chemoradiation or radiation only. However, this strategy results in poor survival due to a high locoregional failure rate (40–60%) (6,7). Furthermore, functional results after definite chemoradiation are often poor (8,9).
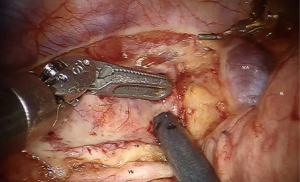
The limitations of conventional open surgical techniques may be overcome by robot-assistance. In 2003 the robot assisted thoraco-laparoscopic minimally invasive esophagectomy (RAMIE) was developed. This technique was demonstrated to be feasible, safe and effective with a high radical resection rate and lymph node yield for patients with resectable esophageal carcinoma (10,11). Robot-assistance provides an enlarged, three-dimensional field of view and facilitates dissection through articulating instruments allowing seven degrees of freedom and tremor filtering (12). With good overview and reach of the thoracic inlet (Figure 2) in combination with extensive experience with RAMIE we postulated that patients with esophageal carcinoma extending into the upper mediastinum could be treated by robot assisted esophageal resection with curative intent.
The aim of this study was to evaluate the surgical and oncological outcomes of RAMIE in this group of patients.
Methods
Patients
Patients with resectable esophageal cancer extending into the proximal 1/3 part of the esophagus (cranial tumor border at 18–24 cm from the incisors) or resectable paratracheal lymphadenopathy (level 2 and/or 4) undergoing potentially curative robot assisted thoraco-laparoscopic esophageal resection at the University Medical Center Utrecht between May 2007 and July 2016 were identified from a prospective database. Patients with distant metastasis were excluded. Institutional Review Board approval was obtained and informed consent requirement was waived for this study (MERC UMC Utrecht ID041, approval number 13-061/C).
Baseline characteristics
The prospectively collected baseline characteristics included sex, age, BMI, medical history and clinical tumor characteristics. Routine diagnostic work up was recorded prospectively; including the use and results of upper gastro-intestinal endoscopy, endoscopic ultrasound, CT-scan of the thorax and abdomen and ultrasonography of the neck region. When indicated, a PET scan, bronchoscopy with endobronchial ultrasound, and/ or fine needle aspiration of suspicious lymph nodes was used. Before start of treatment all patients were discussed at a multidisciplinary tumor board meeting.
Neoadjuvant treatment
Neoadjuvant treatment was administered according to Dutch guidelines. Before May 2012 patients with resectable PCC were treated with chemoradiation according the CROSS schedule (13) and patients with resectable adenocarcinoma were treated with chemotherapy according to the MAGIC (14) schedule. After May 2012 patients were treated with chemoradiation according to the CROSS schedule. Patients with a T4b carcinoma were treated with an extended chemoradiation schedule (Carboplatin AUC 50 mmg/m2 weekly, 6×, concomitant with 50.4 Gy) (15).
Operative procedure
Robot assisted thoraco-laparoscopic esophagectomy with two field lymphadenectomy was performed as previously described (11). Surgery was performed with the patient in left lateral decubitus position, tilted 45° towards prone. During the robot assisted thoracoscopic phase mobilization of the esophagus was combined with a thoracic lymphadenectomy, which included the right and left paratracheal lymph nodes (station 2R, 2L), tracheobronchial lymph nodes (station 4R, 4L), paraaortic nodes (station 6), subcarinal nodes (station 7), peri-esophageal nodes (station 8) and pulmonary ligament nodes (station 9).
Hereafter the patient was positioned in supine position to facilitate laparoscopic gastric mobilization and abdominal lymphadenectomy, which included right and left cardiac lymph nodes (stations 1 and 2), the lesser omental lymph nodes (station 3), left gastric artery nodes (station 7), celiac artery nodes (station 9), root portion of the hepatic and splenic artery (stations 8 and 11), infra-diaphragmatic nodes (station 19) and lymph nodes in the esophageal hiatus of the diaphragm (station 20). After gastric tube formation a cervical hand sewn end-to-side esophagogastrostomy was constructed. The video “Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum” (Figure 3) demonstrates the procedure.

Characteristics of surgery, including operation time, conversion, blood loss, and intra-operative complications were registered prospectively.
Pathological analysis
The resected specimen was evaluated using a standard protocol (11). The pathology report included tumor type, grade, length, invasiveness into the esophageal wall, radicality of the resection (R0 margins not involved, R1 microscopic tumor residual in resection margin, R2 macroscopic tumor residual in resection margin), Mandard score and lymph node status. Stage grouping took place according to the 7th edition of the International Union Against Cancer (UICC) (17).
Postoperative management
After surgery, patients were transferred to the intensive care unit (ICU) where they were extubated on the same day. The first postoperative day, patients were transferred to the medium care unit (MCU) and on the second postoperative day patients were transferred to the ward, unless otherwise indicated. Enteral tube feeding by a needle-catheter jejunostomy was started on the first postoperative day. Oral intake was started between the 5th–7th postoperative day when there were no signs of anastomotic leakage. Patients started with water which was gradually expanded to solid food in case no problems were encountered.
Postoperative complications
All postoperative complications (e.g., anastomotic leakage, pneumonia, chyle leakage, recurrent nerve injury, cardiac events, wound infection, thromboembolic events) were prospectively registered after discussion in a weekly consensus meeting between surgeons and researchers. Pneumonia was defined according to the Uniform Pneumonia Score (18,19). Anastomotic leakage was defined as saliva leakage from the cervical wound and/or radiological signs of anastomotic leakage (contrast leakage, mediastinal fluid/air levels, signs of anastomotic leakage during endoscopy, re-operation or postmortal investigation. Chyle leakage was defined as a triglyceride level in pleural effusion vs. plasma ratio >10. Complication severity was classified using the Modified Clavien Dindo Classification (MCDC) and in-hospital mortality, 30-day mortality and 90-day mortality were registered.
Follow-up
According to the standard follow up regimen described in the Dutch guidelines, routine postoperative outpatient department visits were scheduled at 3, 6, 9 and 12 months postoperatively in the 1th year, bi-annually in the 2nd year and annually in the 3rd, 4th and 5th year. In case of symptoms of tumor recurrence, patients underwent a CT or PET-CT of the thorax and abdomen and if indicated upper gastro-intestinal endoscopy. All patients had at least 1 month of follow up and were followed up till 5 years postoperatively.
Statistical analysis
Statistical analysis was performed using SPSS version 21 (SPSS, Chicago, IL, USA). All continuous data were presented as medians with range, and categorical data as absolute numbers (%). Survival time was calculated as the duration from the day of surgery to death or the last date of follow-up. Disease-free interval was calculated from the day of surgery to the day of definitive diagnosis of recurrent tumor. All patients were evaluated and included in the final analyses.
Results
From May 2007 until July 2016, 31 consecutive patients with upper esophageal cancer underwent robot assisted thoraco-laparoscopic esophagectomy.
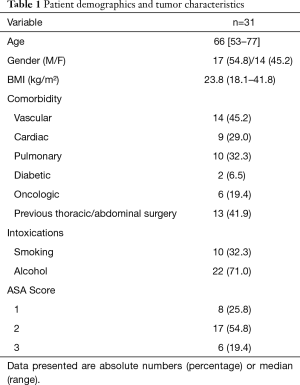
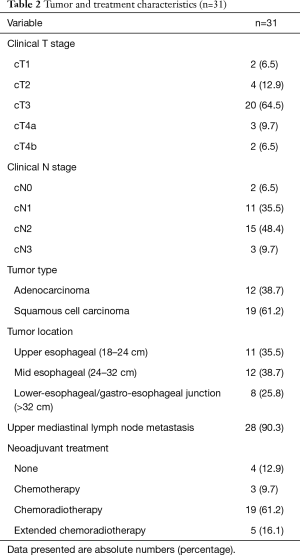
Patients included 17 (55%) men and 14 (45%) women, with a median age of 66 years (range 53–77 years). Comorbidity was present in 19 (62%) patients. Of these patients 6 (19%) were classified ASA 3 (Table 1). cT3 disease was observed in 20 (64%) patients, 3 (10%) patients presented with cT4a disease and 2 (7%) with cT4b disease. The majority of patients (94%) had clinically positive nodal disease (Table 2). Esophageal cancer extended into the upper 1/3 part of the esophagus in 11 (35%) patients, of whom 9 patients also had clinically positive upper mediastinal lymph nodes. All of the remaining 20 patients with mid or lower esophageal carcinoma had clinically positive upper mediastinal lymph nodes, paratracheal level 2 and/or 4. Most patients (57%) had T3N+ disease (Table 3).
Full table
Full table
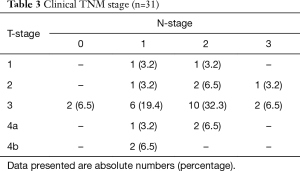
Full table
Neo-adjuvant treatment
Neo-adjuvant treatment consisted of chemotherapy according to the MAGIC schedule (14) in 3 (10%) patients, chemoradiation according to the CROSS schedule (5,13) in 19 (61%) and an extended chemoradiation schedule in 5 (16%) patients (Table 2). The reasons for the extended chemoradiation schedule were stage cT4b carcinoma in 2 (6%) patients, which became resectable tumors after chemoradiation, and ineligibility for surgery in the referring hospital due to of PET-scan positive lymph nodes in the upper mediastinum in 3 patients (10%). Neo-adjuvant treatment was not administered to 4 (13%) patients, reasons being: kidney disease, cardiac disease, refusal of neo-adjuvant treatment and not registered.
Intra-operative results
The median duration of the total procedure was 435 minutes (range 299–874 minutes) and of the thoracoscopic phase 148 minutes (range 105–307 minutes) (Table 4). In 2 (6%) patients conversion to thoracotomy was necessary, the reasons were: pulmonary adhesions disabling trocar placement and adherent relation of the tumor to the aortic arch. One patient needed extra port placement because of a non-compliant lung prohibiting the use of one of the assistant trocars and in one patient a pulmonary defect due to adhesiolysis was oversewn.
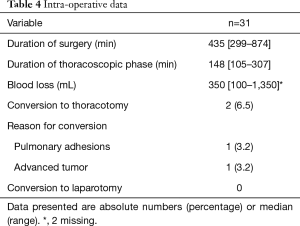
Full table
Postoperative results
An uncomplicated postoperative course was encountered in 7 (23%) patients. Complications MCDC grade 2 occurred in 13 (42%) patients and MCDC grade 3b and higher occurred in 11 (35%) patients. The most frequent complications were pulmonary; 10 (32%) patients were diagnosed with a pneumonia, resulting in ICU readmission for respiratory support in 4 patients (Table 5). Leakage of the cervical esophagogastrostomy occurred in 6 (19%) patients of whom 5 had an intrathoracic manifestation requiring mediastinal drainage. Other complications requiring re-operation in an additional 4 (13%) patients were: a temporary tracheostomy and thoracoscopy for a pseudomonas pneumonia, evacuation of an abdominal wall abscess caused by jejunostomy leakage, gastric tube resection due to necrosis and a tracheo-neo-esophageal fistula. Vocal cord paralysis occurred in 4 (13%) patients which was temporary in 3 out of 4 patients. The median ICU stay was 1 day (range 1–65 days) and hospital stay was 15 days (range 10–118 days). In hospital mortality occurred in 3 (10%) patients. Thirty-day mortality was 2 (6%), 60-day mortality was 3 (10%). Causes of mortality were tracheo-neo-esophageal fistula, sepsis after abdominal wall drainage due to leakage of the jejunal fistula resulting in respiratory and kidney failure, after which refraining further treatment resulting in death, and irreversible ARDS in a patient with COPD Gold III needing extracorporeal life support. Ninety-day mortality was 13% (4 patients); 1.5 months after surgery a patient was readmitted with an abdominal sepsis after re-positioning of the jejunal fistula resulting in multi organ failure. During laparotomy liver metastasis were found, after which treatment was ceased.
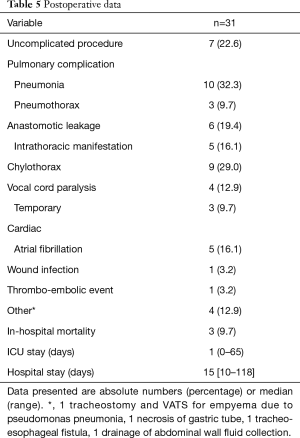
Full table
Histopathological results
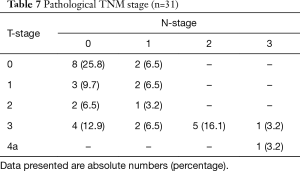
An overview of the histopathological results is shown in Tables 6 and 7. A radical resection (R0) was achieved in 30 (97%) patients and in 1 patient a microscopically irradical (R1) resection was performed. This single irradical resection was in a patient with an uT4a(pleura)N2 adenocarcinoma, with a positive resection margin at the site of tumor ingrowth in a large adjacent artery. A pathological complete response (pCR) of tumor and lymph nodes after neoadjuvant therapy was seen in 7 (23%) patients. The median number of retrieved lymph nodes was 22 [9–57], with a median of 0 positive lymph nodes (range 0–12). Positive lymph nodes were found in 14 (45%) patients.
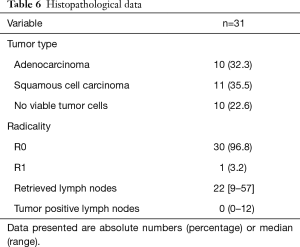
Full table
Full table
Recurrence and outcome
At the time of analysis median time of follow up was 18 months (range 3–81 months). Median disease-free survival was 13 months (range 0–81 months) and median overall survival was 16 months (range 0–81 months). Kaplan Meier curves are shown in Figure 4. Tumor recurrence occurred in 15 (48%) patients and was locoregional only in 3 (10%) patients, systemic only in 5 (16%) patients and combined locoregional and systemic in 7 (23%) patients. At time of analysis 11 (35%) patients were alive, none of these patients had signs of tumor recurrence or metastasis.
Discussion
This study shows that robot assisted thoraco-laparoscopic esophagectomy is feasible in patients with an esophageal carcinoma that involves the upper 1/3 of the esophagus or paratracheal lymph nodes. These patients are often precluded from surgery due to technical difficulties in the dissection at the thoracic inlet (5-7). Short term oncological outcome in terms of radicality and lymph node yield was good, however postoperative morbidity was significant, which emphasizes the need for strict patient selection.
The benefits of robot assisted surgery for this patient group were found in oncological outcome. A high rate of radical (R0) resections (97%) was achieved using robot assisted thoraco-laparoscopic esophagectomy, even though the majority of patients had a T3 or even T4a/b tumor. Robotic assistance provides an excellent overview of the thoracic inlet via a magnified 3-dimensional view. Precise and meticulous dissection is facilitated by articulating surgical instruments (12). Ergonomy is achieved even though the surgery is performed in the confined, hard-to-reach upper thoracic cavity. These advantages enabled a proper mediastinal lymph node dissection including resection of paratracheal lymph nodes, with a median of 22 resected lymph nodes.
The present results are in line with results of other studies on esophagectomy for advanced esophageal carcinoma. Overall complications MCDC grade 3b and higher occurred in 36% of the patients, which is within the range of 31–36% reported previously following esophagectomy (20-23). The pneumonia rate (32%) was comparable to pneumonia rates reported by recent studies that used the same definition (28–31%) (18,24), and within the range reported by other studies on RAMIE (6–45%) (12). The rates of temporary and permanent vocal cord paralysis were also similar to those reported previously following RAMIE (12).
The anastomotic leakage rate (19%) was equal to the previously reported rate following RAMIE (19%) (11). However, intrathoracic manifestations of leakage were seen more often in the present study. This is explained by the extensive dissection of the upper mediastinum that is required to achieve a radical resection and complete station 2 and 4 lymph node dissection in these patients. In hospital mortality was 10%, which is comparable to studies on patients with advanced esophageal cancer (25). However, these rates are higher than in the patients with esophageal cancer located in the mid or lower esophagus. In our series of 108 patients treated by RAMIE mortality was 5% (11). This reflects the different group of patients that were treated in this series.
Co-morbidity was present in majority of our patients, including 6 (19%) patients with an ASA 3 score and 10 (32%) patients with COPD (2 of them with an ASA 3 score). In patients with co-morbidity the risk of perioperative morbidity and mortality is increased. This might be an explanation for the morbidity and mortality rates and indicates that accurate patient selection for this procedure is necessary to reduce in hospital mortality.
Limitations of this study include the retrospective analysis and limited number of patients, reflecting the low incidence of patients with upper mediastinal esophageal cancer referred for surgery in the Western world. The results of these unique data emphasize the role of surgery, even in this group of patients with advanced tumors at a difficult area in the mediastinum.
In conclusion, RAMIE is feasible in patients with proximal esophageal carcinoma or proximal mediastinal lymph node metastasis, with encouraging oncologic outcome and acceptable postoperative morbidity. A prospective study with accurate patient selection and a homogenous patient population with long term follow up should be performed to confirm these results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional Review Board approval was obtained and informed consent requirement was waived for this study (MERC UMC Utrecht ID041, approval number 13-061/C).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Mariette C, Piessen G. Oesophageal cancer: how radical should surgery be? Eur J Surg Oncol 2012;38:210-3. [Crossref] [PubMed]
- Omloo JM, Law SY, Launois B, et al. Short and long-term advantages of transhiatal and transthoracic oesophageal cancer resection. Eur J Surg Oncol 2009;35:793-7. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Schieman C, Wigle DA, Deschamps C, et al. Salvage resections for recurrent or persistent cancer of the proximal esophagus after chemoradiotherapy. Ann Thorac Surg 2013;95:459-63. [Crossref] [PubMed]
- Miyata H, Yamasaki M, Takiguchi S, et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol 2009;100:442-6. [Crossref] [PubMed]
- Ishikura S, Nihei K, Ohtsu A, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003;21:2697-702. [Crossref] [PubMed]
- Gkika E, Gauler T, Eberhardt W, et al. Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis Esophagus 2014;27:678-84. [Crossref] [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22 Suppl 3:S1350-6. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Meerten EV, Van Rij C, Tesselaar ME, et al. Definitive concurrent chemoradiation (CRT) with weekly paclitaxel and carboplatin for patients (pts) with irresectable esophageal cancer: A phase II study. J Clin Oncol 2010;28:abstr e14508.
- van der Horst S, Weijs TJ, Ruurda JP, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum. Asvide 2017;4:280. Available online: http://www.asvide.com/articles/1589
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- van der Sluis PC, Verhage RJ, van der Horst S, et al. A new clinical scoring system to define pneumonia following esophagectomy for cancer. Dig Surg 2014;31:108-16. [Crossref] [PubMed]
- Weijs TJ, Seesing MF, van Rossum PS, et al. Internal and External Validation of a multivariable Model to Define Hospital-Acquired Pneumonia After Esophagectomy. J Gastrointest Surg 2016;20:680-7. [Crossref] [PubMed]
- Xia BT, Rosato EL, Chojnacki KA, et al. Major perioperative morbidity does not affect long-term survival in patients undergoing esophagectomy for cancer of the esophagus or gastroesophageal junction. World J Surg 2013;37:408-15. [Crossref] [PubMed]
- Goldberg RF, Bowers SP, Parker M, et al. Technical and perioperative outcomes of minimally invasive esophagectomy in the prone position. Surg Endosc 2013;27:553-7. [Crossref] [PubMed]
- Blom RL, Klinkenbijl JH, Hollmann MW, et al. Results of the introduction of a minimally invasive esophagectomy program in a tertiary referral center. J Thorac Dis 2012;4:467-73. [PubMed]
- Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798-807. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Immediate Postoperative Oral Nutrition Following Esophagectomy: A Multicenter Clinical Trial. Ann Thorac Surg 2016;102:1141-8. [Crossref] [PubMed]
- In H, Palis BE, Merkow RP, et al. Doubling of 30-Day Mortality by 90 Days After Esophagectomy: A Critical Measure of Outcomes for Quality Improvement. Ann Surg 2016;263:286-91. [Crossref] [PubMed]