Utility of cardiac magnetic resonance for evaluation of mitral regurgitation prior to mitral valve surgery
Introduction
Mitral regurgitation (MR) is a leading cause of valvular heart disease in the U.S. and globally. Nearly 20% of the U.S. population has some degree of MR, including over 4 million Americans with advanced (moderate or severe) MR (1-3). Whereas causality of MR can vary [e.g., primary valvular degeneration, secondary valvular dysfunction due to left ventricular (LV) remodeling], adverse prognosis conferred by MR is well known: population-based outcomes studies have shown MR to independently confer increased risk for heart failure, arrhythmia, and death (4-7). Poor clinical prognosis conferred by MR is proportionate to severity of valvular dysfunction (8), highlighting the importance of prompt identification and effective therapy to treat MR and reduce its serious clinical sequelae.
Interventional therapies such as mitral valve repair and valve replacement have the potential to reduce or eliminate MR. A key clinical conundrum concerns appropriate timing of MR-directed therapies. Delayed intervention increases pre-operative morbidity and decreases procedural efficacy (9,10), possibly due to impact of LV or left atrial (LA) dilation on mitral apparatus geometry or wall stress (11-13). Accordingly, consensus guidelines recommend surgery for patients with severe primary MR if symptoms are present or, in the case of asymptomatic individuals, if LV dysfunction (ejection fraction <60%) or chamber dilation (end-systolic diameter ≥40 mm) is present (14). Echocardiography (echo) is widely used to guide decision-making concerning timing of interventional therapies for MR (14). However, echo can be suboptimal for this purpose, as image quality can vary (15), chamber quantification is typically predicated on 2-dimensional (2D) geometric assumptions [rather than 3-dimensional (3D) imaging] (16), and MR quantification can be challenging in the context of regurgitant jet eccentricity (17). These limitations may explain recent data suggesting lack of correlation between pre-operative echo-quantified MR severity and LV reverse remodeling after mitral valve surgery (18). Knowledge gaps regarding predictors of procedural success limit the ability to optimize decision-making for patients with MR.
Cardiac magnetic resonance (CMR) can assess MR as well as its predisposing risk factors. A variety of CMR pulse sequences can be used for MR assessment (Table 1). Phase velocity encoded CMR can quantify MR severity based on both direct measures of regurgitant flow through the mitral valve as well as indirect measures of differential stroke volumes (19). Cine-CMR can identify mitral valve alterations (e.g., prolapse, rheumatic disease) as well as secondary changes in the mitral apparatus (e.g., papillary muscle displacement) that pre-dispose to MR (20). More broadly, cine-CMR can quantify changes in LV function and size with high precision so as to guide decision-making for mitral valve interventions. Delayed enhancement CMR (DE-CMR) enables highly accurate assessment of myocardial infarction (MI) within LV myocardium underlying the mitral valve—a known causal substrate for MR (21,22). This article will review established literature concerning utility of CMR for evaluation of MR severity and causality, as well as emerging data concerning utility of CMR for predicting MR response to therapeutic interventions.

Full table
Quantification of MR
CMR provides a variety of approaches to measure MR severity. These include quantification via 2D or time resolved multidimensional (4D) flow quantification, as well as semi-quantitative assessment via cine-CMR.
2D phase contrast (PC) velocity encoded CMR can measure MR via several approaches. A common method calculates MR based on differential forward stroke volume between the mitral valve and aortic valve, which can each be calculated via PC-CMR sampling at the respective valve orifices (23). One pitfall of this approach stems from translational valve motion, which can produce under-sampling of flow and produce errors in MR quantification (19). A variant of this approach employs PC-CMR to sample flow through the aortic valve (a region with lesser translational motion than the mitral valve), and cine-CMR to measure LV stroke volume (∆ end-diastole − end-systole); differential stroke volume corresponds to the amount of MR (Figure 1) (24). This method has been shown to correlate well with invasive measurements of MR via cardiac catheterization, as well as non-invasive measurement via echo (25-27). MR as quantified based on differential aortic and mitral valve forward stroke volume can be affected by concomitant aortic regurgitation. Another potential limitation stems from cine-CMR derived LV stroke volume, which can be challenging in the context of arrhythmias and yield slight variability in the setting of prominent LV trabeculations (28,29). PC data can also be used to calculate MR as differential stroke volume between the left and right ventricle (RV); in the absence of regurgitant valve disease or intracardiac shunts, LV and RV stroke volumes should be near identical (24). Key limitations of this approach include the fact that it is only valid in the setting of isolated MR, and can suffer from pitfalls due to above noted sources of variability with respect to cardiac chamber contouring. An alternative to stroke volume based methods entails use of phase velocity encoded imaging to directly measure MR based on sampling of the regurgitant jet: while this measure would be expected to work well in the context of central jets, it can be challenging in the context of multiple or eccentric MR jets (30).
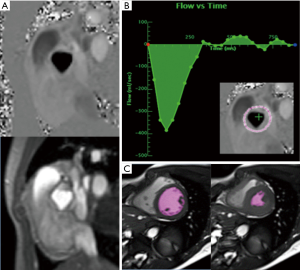
Time resolved multidimensional (4D) velocity encoded flow CMR is an emerging method that holds the potential to quantitatively assess MR irrespective of jet directionality. Using this approach, velocity field vectors are measured in x-, y-, and z-directions, rather than in a single plane as can be offered via 2D PC imaging (31). 4D flow imaging is typically acquired free breathing (via navigator gating or other sampling methods), allowing flow data to be acquired with high spatial resolution (32,33). To date, 4D flow has been primarily used to assess vascular flow, such as aortic anomalies and congenital heart disease. However, emerging data has suggested that 4D flow may provide utility for MR assessment. Among a cohort of 64 patients with functional MR undergoing multidimensional PC-CMR and 3D echo, Marsan et al. reported excellent correlations (r=0.94, P<0.001) between mitral regurgitant volumes measured by the two modalities (34). Similarly, among 32 patients with atrioventricular septal defects, Calkoen et al. reported 4D flow to yield strong correlation with conventional 2D CMR quantification based on differential stroke volume (r=0.97, P<0.001) (35). These data build upon earlier pilot studies supporting use of 4D flow for MR assessment (36,37). Challenges of 4D flow application in current clinical practice include prolonged acquisition times (typically 5–10 minutes) as well as requisite pre-determination of peak sample velocities (for which inaccuracies can produce aliasing and limit data utility) (31). More broadly, further clinical studies are needed to demonstrate incremental utility of 4D flow vs. conventional methods, paralleled by technical advances to reduce acquisition time in order to facilitate widespread use of this powerful technique in clinical practice.
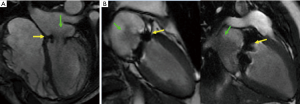
Cine-CMR [steady-state free precession (SSFP)] imaging provides an additional means by which MR can be assessed. Central to this approach is the observation that flow turbulence (e.g., due to MR jets) produces spin-spin dephasing, which can be visualized on routine SSFP images (Figure 2). MR can thus be semi-quantitatively assessed based on jet size or depth (i.e., extent of dephasing) in relation to the left atrium (38). It is important to note that cine-CMR provides a semi-quantitative means of assessing MR, as opposed to quantitative approaches as provided by PC-CMR (39). However, a key advantage concerns the fact that necessary cine-CMR data to assess MR is encompassed in nearly all routine CMR exams and thus requires no tailored imaging (i.e., no additional patient breath holds or prolonged scanner time). MR assessment based on cine-CMR spin-spin dephasing has been validated in several cohorts. Among a cohort of 68 patients who underwent CMR and echo (median interval 2 days), Heitner et al. reported moderate agreement between MR as graded by cine-CMR and echo [kappa=0.47 (0.29–0.65)] (38). In a study of 33 patients with a prosthetic mitral valve undergoing both CMR and transesophageal echo (median interval 2 days), Simprini et al. similarly reported moderate agreement in MR grade (kappa=0.44) when calculated by the two modalities, with the majority of discordances differing by ≤1 MR grade (40). Another study of 44 patients with MR likewise found cine-CMR graded MR severity to moderately correlate (r=0.66, P<0.001) with quantitative MR assessment as measured by differential stroke volumes (39). Given that cine-CMR data for visual assessment of MR is intrinsic to near all exams, this approach is often used as an adjunct to phase-velocity encoded quantification, for which it can provide important additive data regarding jet directionality and origin.
Elucidation of MR etiology
Primary MR
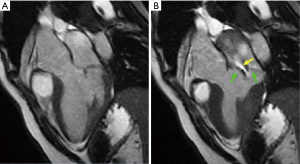
Primary MR stems from abnormalities in the mitral valve or its immediate supporting structures (i.e., mitral annulus, chordae tendineae, or papillary muscles). CMR provides high spatial resolution imaging and excellent endocardial definition, allowing for localization of structural abnormalities responsible for mitral valve incompetence (41). Like echo, cine-CMR can assess valve morphology—including rheumatic deformation as well as mitral valve prolapse (MVP) (Figure 3). Localization of valve dysfunction is important for surgical planning, as some mitral valve abnormalities are less amenable to successful mitral valve repair (e.g., repair is less durable for extensive anterior leaflet disease) (42). Among a cohort of 25 patients with MVP, Han et al. reported cine-CMR assessment of leaflet displacement to provide high sensitivity (100%) and specificity (100%) compared to the reference standard of transthoracic echo (43). Among a cohort of 27 patients with MVP, Gabriel et al. reported that cine-CMR accurately determined presence or absence of involvement of each leaflet in 98% of cases compared to the reference of transthoracic echo; in a subset of patients (n=10) who underwent transesophageal echo or surgery, accuracy of cine-CMR was 82% (44). Cine-CMR can also identify geometric alterations in the mitral apparatus that correlate with MR severity. Among 71 patients with MVP, cine-CMR evidenced anterior mitral valve leaflet length, posterior leaflet displacement, and posterior leaflet thickness each increased in relation to severity of MR (45). Other studies have used PC-CMR to localize MVP-associated valve deformities. Among a mixed cohort of patients with MVP and normative controls, Han et al. reported MVP to be associated with increased peak papillary muscle systolic velocity and maximum papillary muscle excursion (46). Taken together, these data indicate that both cine-CMR and PC-CMR provide utility for physiologic localization of mitral apparatus abnormalities among patients with MVP.
Beyond mitral valve structure and function, CMR can be used to assess altered myocardial tissue properties associated with MVP. For example, Han et al. used DE-CMR to assess papillary muscle fibrosis among a pilot cohort of 16 patients with MVP (43). The authors reported papillary fibrosis to be present in over half (10/16) patients with MVP in whom it was associated with increased ventricular ectopy, suggesting that this finding may be of prognostic significance (43). Further studies are warranted to test prevalence of MVP-associated papillary fibrosis among larger (e.g., multicenter) cohorts, as well as to test the predictive value of this finding for stratifying actual clinical outcomes among patients with MVP.
Primary MR can also stem from acquired conditions, including rheumatic heart disease and infective endocarditis (IE). Cine-CMR can be used to identify vegetations in patients with known or suspected IE. However, one limitation of this approach concerns the fact that temporal resolution of most CMR techniques is lower than that of echo, meaning that highly mobile vegetations can potentially be missed. As evidence of this, Zatorska et al. used CMR and echo to study 20 consecutive patients with IE; only 77% of echo-evidenced vegetations were detected by CMR (47). Other groups have focused use of CMR on secondary complications of IE, including mitral valve annular abscess (48) and mitral valve aneurysm (49). Some studies have suggested that hyperenhancement in association with valvular lesions may indicate inflammation and thus aid in the diagnosis of IE (47,50); however, limited direct correlations with histopathology and/or systemic markers of inflammation limit validation of this approach, highlighting the fact that further studies are needed in this area.
Functional MR
Secondary (functional) MR is a common cause of MR and occurs as a consequence of LV chamber remodeling. Often occurring in patients who have sustained inferior wall MI, the primary mechanism is thought to be papillary muscle displacement secondary to ventricular dilation, resulting in increased tethering force on the mitral valve leaflets, which prevents adequate leaflet coaptation during systole (51). CMR provides high spatial resolution that can detail mitral valve geometry among patients with secondary MR, for whom image analyses hold the potential to guide surgical planning. This concept was illustrated in a study by Kaji et al., for which CMR was performed in 38 patients with prior inferior or posterior MI (52). Results demonstrated increased septal-lateral and inter-commissural diameters, as well as differences in annular geometry, among patients who subsequently developed MR. Similarly, in a pig model with induced chronic ischemic MR, CMR measurements of annular area, septal-lateral distance, and commissure-commissure distance were all increased (P<0.05) in the context of MR (53). These findings support the notion that CMR can be used to localize geometric causality of secondary MR, allowing for focused surgical or percutaneous reparative approaches to address underlying MR mechanism.
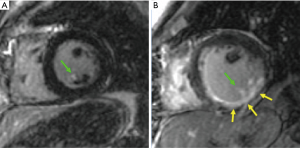
Beyond functional imaging and direct MR assessment, CMR enables identification of tissue properties that can contribute to ischemic MR. CMR can identify infarction within the papillary muscles as well as underlying LV myocardium (Figure 4), and has been used to study differential impact of MI in each region on MR: in some studies, papillary muscle infarction (PMI) has been associated with MR (22), whereas other studies have largely found no such association (21,54,55). Our group investigated the relative impact of PMI on MR, finding that PMI was closely linked to MI in the underlying LV chamber wall, which was the primary determinant of MR severity (21). Three separate studies—encompassing a total of 1,009 patients—have demonstrated PMI on CMR to not be associated with MR (21,54,55). These data are of central importance with respect to mechanistic causality of functional MR, supporting the notion that dysfunction of the myocardium underlying the papillary muscles (rather than the papillary muscles themselves) produces mitral valvular incompetence and determines therapeutic response. As support of this, in a study of patients undergoing concomitant surgical revascularization and mitral annuloplasty for ischemic MR, those with a high burden of CMR-detected scar in the region of the posterior papillary muscle were more likely to exhibit recurrence of advanced MR post-operatively (56).
Prediction and monitoring of MR therapeutic response
Limited data exists with respect to utility of CMR tissue characterization for direct prediction of MR response to interventional therapies. However, a wide array of data has shown MR improvement to be linked to LV reverse remodeling (57-59). In this regard, the predictive utility of viability assessment via CMR is well established: transmural extent of viable myocardium on CMR is well known to predict LV reverse remodeling following surgical or percutaneous (PCI) revascularization (60). For example, among a cohort of 50 CAD patients undergoing elective coronary revascularization, Kim et al. showed that dysfunctional but viable myocardium on pre-procedural DE-CMR predicted post-revascularization recovery of LV function (61). Other studies have shown viability assessment via DE-CMR to predict reduction in LV chamber size among patients undergoing elective coronary bypass graft surgery, as well as among patients undergoing PCI (60,62,63). Of interest, absence of viable myocardium within the mitral apparatus has been shown to stratify likelihood of MR following acute MI, suggesting this approach may predict longitudinal risk for MR in post-MI patients being considered for coronary revascularization (21). As CMR is a highly reproducible and well-validated method for the evaluation of MI and ischemia (64,65), it holds the potential to serve as a predictive tool for identification of patients in whom MR will improve or resolve in response to revascularization of LV myocardium underlying the mitral valve.
CMR has also been shown to provide utility for monitoring of therapeutic response to percutaneous or surgical mitral valve interventions. Among patients who have undergone surgical mitral valve replacement, Simprini et al. showed cine-CMR assessment of MR severity to be highly sensitive for identification of residual MR relative to transesophageal echo (40). Similarly, CMR has been shown to identify reverse LV remodeling among patients who have undergone percutaneous mitral repair: Among a cohort of 27 patients with advanced (≥ moderate) MR, Krumm et al. reported cine-CMR to be feasible for the visual assessment of MR following the procedure (i.e., associated with minimal image artifact) (66). Moreover, the authors demonstrated cine-CMR to be capable of identifying post-procedural LV reverse remodeling, as evidenced by serial reductions in LV and LA chamber size (P<0.01) and improvements in LV ejection fraction (P=0.004) following device implantation. Given the fact that CMR entails no radiation and (unlike transesophageal echo) is non-invasive, these data support its utility as a preferred approach for assessment of post-procedural response in patients for whom transthoracic echo results are limited or non-diagnostic.
Recent data has examined whether CMR-based MR assessment provides incremental utility for predicting LV reverse remodeling following mitral valve interventions. Among a cohort of patients undergoing CMR and echo prior to primary mitral valve surgery, Uretsky et al. reported a strong correlation between post-surgical LV remodeling and MR severity as assessed by CMR (r=0.85, P<0.0001) but no correlation between post-surgical LV remodeling and MR severity as assessed by echo (r=0.32, P=0.1) (18). While it is tempting to use this data as a basis for employing CMR as the primary test to assess MR and predict therapeutic response to mitral valve surgery, it should be noted that sample size of patients with post-surgical follow-up was small [n=26; 25% (26/103) of total study population], etiology of MR varied, and duration between pre-procedural CMR and echo was non-standardized [median 15 days (IQR 7, 35)]—suggesting that differences between tests may stem from population variance or intrinsic variability in MR, and that better agreement would have been found via imaging tests performed for primary research purposes near simultaneously. Given that echo is widely available, well-validated, and far less expensive than CMR, further studies are warranted to better identify specific MR populations in whom procedural decision-making regarding mitral valve interventions should primarily be guided by CMR findings.
Computational modeling
Beyond primary image interpretation, CMR can be analyzed via computational approaches so as to maximize its utility as a diagnostic and prognostic tool. Different computational approaches have been used for this purpose, including machine learning as well as finite element (FE) modeling. In FE modeling, the structure of interest is divided into discrete, interconnected elements, enabling the determination of stress-strain relationships. Several studies have used FE modeling to provide insight into functional and geometric alterations that occur following surgical intervention (67,68). Using CMR images obtained in a sheep with moderate MR following posterobasal MI, Wenk et al. created an FE model of the LV, mitral apparatus, and chordae tendineae (69). The model was able to identify the existence of MR. Further, reduction of infarct stiffness in the model was associated with papillary muscle displacement and a 33% increase in the gap between the mitral leaflets, consistent with increased MR (69). Computational modeling has been applied to patients undergoing mitral valve repair as well. In a single patient with posterior leaflet prolapse, Ge et al. created an FE model of the LV and mitral apparatus pre-operatively using CMR and 3D transesophageal echo; mitral valve repair was then simulated (70). FE modeling was able to accurately predict changes in mitral annulus dimensions and leaflet coaptation, supporting a role for this approach for prediction of post-operative changes in the mitral apparatus (70). FE modeling has also been used to investigate stress distribution over the mitral leaflets following different types of repair. In the aforementioned patient with posterior leaflet prolapse, FE modeling was used to simulate both neochord placement and triangular leaflet resection (71). Modeling demonstrated decreased leaflet stress with neochord placement when compared to leaflet resection, suggesting improved durability with neochord placement (71). Studies are ongoing to further elucidate the ability of CMR-based FE models to predict outcomes in patients with MR undergoing intervention. In particular, models using CMR data on infarct and ischemia distribution are being developed to identify patients that will have a decrease in MR following revascularization alone, which will optimize selection of surgical procedure.
Conclusions
CMR has been well validated as an accurate diagnostic tool for assessment of MR severity and etiology. Flow quantification via CMR enables assessment of subtle changes in MR, whereas functional imaging can quantify associated cardiac chamber remodeling with excellent precision. Tissue characterization via CMR (i.e., infarct assessment) enables insights into tissue based substrate for MR, and has been shown to predict LV remodeling response to coronary revascularization as well as risk for MR in selected (post-MI) cohorts. Further studies are ongoing to test incremental utility of CMR in comparison to conventional echo assessment, as well as to guide decision-making for patients being considered for directed therapies to regress or resolve MR.
Acknowledgements
Funding: This work was funded in part by National Institutes of Health grant #1R01HL128278-01 (PI: JW Weinsaft, MD).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol 2001;87:298-304. [Crossref] [PubMed]
- Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897-902. [Crossref] [PubMed]
- Bureau USC. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios. April 1, 2010 to July 1, 2015.
- Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996;335:1417-23. [Crossref] [PubMed]
- Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol 2002;40:84-92. [Crossref] [PubMed]
- Tribouilloy C, Rusinaru D, Grigioni F, et al. Long-term mortality associated with left ventricular dysfunction in mitral regurgitation due to flail leaflets: a multicenter analysis. Circ Cardiovasc Imaging 2014;7:363-70. [Crossref] [PubMed]
- Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675-80. [Crossref] [PubMed]
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [Crossref] [PubMed]
- Kang DH, Kim JH, Rim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation 2009;119:797-804. [Crossref] [PubMed]
- Enriquez-Sarano M, Suri RM, Clavel MA, et al. Is there an outcome penalty linked to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc Surg 2015;150:50-8. [Crossref] [PubMed]
- Enriquez-Sarano M, Basmadjian AJ, Rossi A, et al. Progression of mitral regurgitation: a prospective Doppler echocardiographic study. J Am Coll Cardiol 1999;34:1137-44. [Crossref] [PubMed]
- Beeri R, Yosefy C, Guerrero JL, et al. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: a functional and molecular study. Circulation 2007;116:I288-93. [Crossref] [PubMed]
- Beaudoin J, Levine RA, Guerrero JL, et al. Late repair of ischemic mitral regurgitation does not prevent left ventricular remodeling: importance of timing for beneficial repair. Circulation 2013;128:S248-52. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Enriquez-Sarano M, Freeman WK, Tribouilloy CM, et al. Functional anatomy of mitral regurgitation: accuracy and outcome implications of transesophageal echocardiography. J Am Coll Cardiol 1999;34:1129-36. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Enriquez-Sarano M, Tajik AJ, Bailey KR, et al. Color flow imaging compared with quantitative Doppler assessment of severity of mitral regurgitation: influence of eccentricity of jet and mechanism of regurgitation. J Am Coll Cardiol 1993;21:1211-9. [Crossref] [PubMed]
- Uretsky S, Gillam L, Lang R, et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol 2015;65:1078-88. [Crossref] [PubMed]
- Polte CL, Bech-Hanssen O, Johnsson AA, et al. Mitral regurgitation quantification by cardiovascular magnetic resonance: a comparison of indirect quantification methods. Int J Cardiovasc Imaging 2015;31:1223-31. [Crossref] [PubMed]
- Chan KM, Wage R, Symmonds K, et al. Towards comprehensive assessment of mitral regurgitation using cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2008;10:61. [Crossref] [PubMed]
- Chinitz JS, Chen D, Goyal P, et al. Mitral apparatus assessment by delayed enhancement CMR: relative impact of infarct distribution on mitral regurgitation. JACC Cardiovasc Imaging 2013;6:220-34. [Crossref] [PubMed]
- Okayama S, Uemura S, Soeda T, et al. Clinical significance of papillary muscle late enhancement detected via cardiac magnetic resonance imaging in patients with single old myocardial infarction. Int J Cardiol 2011;146:73-9. [Crossref] [PubMed]
- Fujita N, Chazouilleres AF, Hartiala JJ, et al. Quantification of mitral regurgitation by velocity-encoded cine nuclear magnetic resonance imaging. J Am Coll Cardiol 1994;23:951-8. [Crossref] [PubMed]
- Krieger EV, Lee J, Branch KR, et al. Quantitation of mitral regurgitation with cardiac magnetic resonance imaging: a systematic review. Heart 2016;102:1864-70. [Crossref] [PubMed]
- Cawley PJ, Hamilton-Craig C, Owens DS, et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging 2013;6:48-57. [Crossref] [PubMed]
- Hundley WG, Li HF, Willard JE, et al. Magnetic resonance imaging assessment of the severity of mitral regurgitation. Comparison with invasive techniques. Circulation 1995;92:1151-8. [Crossref] [PubMed]
- Shanks M, Siebelink HM, Delgado V, et al. Quantitative assessment of mitral regurgitation: comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging 2010;3:694-700. [Crossref] [PubMed]
- Weinsaft JW, Cham MD, Janik M, et al. Left ventricular papillary muscles and trabeculae are significant determinants of cardiac MRI volumetric measurements: effects on clinical standards in patients with advanced systolic dysfunction. Int J Cardiol 2008;126:359-65. [Crossref] [PubMed]
- Simprini LA, Goyal P, Codella N, et al. Geometry-independent inclusion of basal myocardium yields improved cardiac magnetic resonance agreement with echocardiography and necropsy quantified left-ventricular mass. J Hypertens 2013;31:2069-76. [Crossref] [PubMed]
- Myerson SG, Francis JM, Neubauer S. Direct and indirect quantification of mitral regurgitation with cardiovascular magnetic resonance, and the effect of heart rate variability. MAGMA 2010;23:243-9. [Crossref] [PubMed]
- Dyverfeldt P, Bissell M, Barker AJ, et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015;17:72. [Crossref] [PubMed]
- Markl M, Harloff A, Bley TA, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007;25:824-31. [Crossref] [PubMed]
- Uribe S, Beerbaum P, Sorensen TS, et al. Four-dimensional (4D) flow of the whole heart and great vessels using real-time respiratory self-gating. Magn Reson Med 2009;62:984-92. [Crossref] [PubMed]
- Marsan NA, Westenberg JJ, Ypenburg C, et al. Quantification of functional mitral regurgitation by real-time 3D echocardiography: comparison with 3D velocity-encoded cardiac magnetic resonance. JACC Cardiovasc Imaging 2009;2:1245-52. [Crossref] [PubMed]
- Calkoen EE, Westenberg JJ, Kroft LJ, et al. Characterization and quantification of dynamic eccentric regurgitation of the left atrioventricular valve after atrioventricular septal defect correction with 4D Flow cardiovascular magnetic resonance and retrospective valve tracking. J Cardiovasc Magn Reson 2015;17:18. [Crossref] [PubMed]
- Westenberg JJ, Roes SD, Ajmone Marsan N, et al. Mitral valve and tricuspid valve blood flow: accurate quantification with 3D velocity-encoded MR imaging with retrospective valve tracking. Radiology 2008;249:792-800. [Crossref] [PubMed]
- Roes SD, Hammer S, van der Geest RJ, et al. Flow assessment through four heart valves simultaneously using 3-dimensional 3-directional velocity-encoded magnetic resonance imaging with retrospective valve tracking in healthy volunteers and patients with valvular regurgitation. Invest Radiol 2009;44:669-75. [Crossref] [PubMed]
- Heitner J, Bhumireddy GP, Crowley AL, et al. Clinical application of cine-MRI in the visual assessment of mitral regurgitation compared to echocardiography and cardiac catheterization. PLoS One 2012;7:e40491. [Crossref] [PubMed]
- Reddy ST, Shah M, Doyle M, et al. Evaluation of cardiac valvular regurgitant lesions by cardiac MRI sequences: comparison of a four-valve semi-quantitative versus quantitative approach. J Heart Valve Dis 2013;22:491-9. [PubMed]
- Simprini LA, Afroz A, Cooper MA, et al. Routine cine-CMR for prosthesis-associated mitral regurgitation: a multicenter comparison to echocardiography. J Heart Valve Dis 2014;23:575-82. [PubMed]
- Lopez-Mattei JC, Shah DJ. The role of cardiac magnetic resonance in valvular heart disease. Methodist Debakey Cardiovasc J 2013;9:142-8. [Crossref] [PubMed]
- David TE, Ivanov J, Armstrong S, et al. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130:1242-9. [Crossref] [PubMed]
- Han Y, Peters DC, Salton CJ, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging 2008;1:294-303. [Crossref] [PubMed]
- Gabriel RS, Kerr AJ, Raffel OC, et al. Mapping of mitral regurgitant defects by cardiovascular magnetic resonance in moderate or severe mitral regurgitation secondary to mitral valve prolapse. J Cardiovasc Magn Reson 2008;10:16. [Crossref] [PubMed]
- Delling FN, Kang LL, Yeon SB, et al. CMR predictors of mitral regurgitation in mitral valve prolapse. JACC Cardiovasc Imaging 2010;3:1037-45. [Crossref] [PubMed]
- Han Y, Peters DC, Kissinger KV, et al. Evaluation of papillary muscle function using cardiovascular magnetic resonance imaging in mitral valve prolapse. Am J Cardiol 2010;106:243-8. [Crossref] [PubMed]
- Zatorska K, Michalowska I, Duchnowski P, et al. The Usefulness of Magnetic Resonance Imaging in the Diagnosis of Infectious Endocarditis. J Heart Valve Dis 2015;24:767-75. [PubMed]
- Pasowicz M, Klimeczek P, Wicher-Muniak E, et al. Usefulness of magnetic resonance imaging in diagnosis of mitral valve anulus abscess--case report. Przegl Lek 2002;59:623-5. [PubMed]
- Saghir S, Ivey TD, Kereiakes DJ, et al. Anterior mitral valve leaflet aneurysm due to infective endocarditis detected by cardiac magnetic resonance imaging. Rev Cardiovasc Med 2006;7:157-9. [PubMed]
- Dursun M, Yilmaz S, Yilmaz E, et al. The utility of cardiac MRI in diagnosis of infective endocarditis: preliminary results. Diagn Interv Radiol 2015;21:28-33. [Crossref] [PubMed]
- Otsuji Y, Levine RA, Takeuchi M, et al. Mechanism of ischemic mitral regurgitation. J Cardiol 2008;51:145-56. [Crossref] [PubMed]
- Kaji S, Nasu M, Yamamuro A, et al. Annular geometry in patients with chronic ischemic mitral regurgitation: three-dimensional magnetic resonance imaging study. Circulation 2005;112:I409-14. [PubMed]
- Jensen H, Jensen MO, Ringgaard S, et al. Geometric determinants of chronic functional ischemic mitral regurgitation: insights from three-dimensional cardiac magnetic resonance imaging. J Heart Valve Dis 2008;17:16-22; discussion 3. [PubMed]
- Eitel I, Gehmlich D, Amer O, et al. Prognostic relevance of papillary muscle infarction in reperfused infarction as visualized by cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2013;6:890-8. [Crossref] [PubMed]
- Tanimoto T, Imanishi T, Kitabata H, et al. Prevalence and clinical significance of papillary muscle infarction detected by late gadolinium-enhanced magnetic resonance imaging in patients with ST-segment elevation myocardial infarction. Circulation 2010;122:2281-7. [Crossref] [PubMed]
- Flynn M, Curtin R, Nowicki ER, et al. Regional wall motion abnormalities and scarring in severe functional ischemic mitral regurgitation: A pilot cardiovascular magnetic resonance imaging study. J Thorac Cardiovasc Surg 2009;137:1063-70 e2.
- Penicka M, Linkova H, Lang O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation 2009;120:1474-81. [Crossref] [PubMed]
- De Bonis M, Lapenna E, Verzini A, et al. Recurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathy. Ann Thorac Surg 2008;85:932-9. [Crossref] [PubMed]
- Gelsomino S, Lorusso R, Capecchi I, et al. Left ventricular reverse remodeling after undersized mitral ring annuloplasty in patients with ischemic regurgitation. Ann Thorac Surg 2008;85:1319-30. [Crossref] [PubMed]
- Glaveckaite S, Valeviciene N, Palionis D, et al. Prediction of long-term segmental and global functional recovery of hibernating myocardium after revascularisation based on low dose dobutamine and late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2014;16:83. [Crossref] [PubMed]
- Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445-53. [Crossref] [PubMed]
- Kirschbaum SW, Rossi A, Boersma E, et al. Combining magnetic resonance viability variables better predicts improvement of myocardial function prior to percutaneous coronary intervention. Int J Cardiol 2012;159:192-7. [Crossref] [PubMed]
- Skala T, Hutyra M, Vaclavik J, et al. Prediction of long-term reverse left ventricular remodeling after revascularization or medical treatment in patients with ischemic cardiomyopathy: a comparative study between SPECT and MRI. Int J Cardiovasc Imaging 2011;27:343-53. [Crossref] [PubMed]
- Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453-60. [Crossref] [PubMed]
- Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol 2009;55:1-16. [Crossref] [PubMed]
- Krumm P, Zuern CS, Wurster TH, et al. Cardiac magnetic resonance imaging in patients undergoing percutaneous mitral valve repair with the MitraClip system. Clin Res Cardiol 2014;103:397-404. [Crossref] [PubMed]
- Lee LC, Genet M, Dang AB, et al. Applications of computational modeling in cardiac surgery. J Card Surg 2014;29:293-302. [Crossref] [PubMed]
- Hashim S, Richens D. Finite element method in cardiac surgery. Interact Cardiovasc Thorac Surg 2006;5:5-8. [Crossref] [PubMed]
- Wenk JF, Zhang Z, Cheng G, et al. First finite element model of the left ventricle with mitral valve: insights into ischemic mitral regurgitation. Ann Thorac Surg 2010;89:1546-53. [Crossref] [PubMed]
- Ge L, Morrel WG, Ward A, et al. Measurement of mitral leaflet and annular geometry and stress after repair of posterior leaflet prolapse: virtual repair using a patient-specific finite element simulation. Ann Thorac Surg 2014;97:1496-503. [Crossref] [PubMed]
- Morgan AE, Pantoja JL, Grossi EA, et al. Neochord placement versus triangular resection in mitral valve repair: A finite element model. J Surg Res 2016;206:98-105. [Crossref] [PubMed]


