Pleural abrasion should not routinely preferred in treatment of primary spontaneous pneumothorax
Introduction
Primary spontaneous pneumothorax (PSP) is a significant global health problem, with reported yearly incidence of 18.0–28.0 per 100,000 in men and 1.2–6.0 per 100,000 in women (1). The main purpose of PSP treatment is to prevent its recurrence. Though various methods have been used in PSP treatment, including simple aspiration, chest drainage, bullectomy, medical pleurodesis, and surgical pleurodesis (2-4), bullectomy with surgical pleurodesis (abrasion, pleurectomy, surgical chemical pleurodesis, et al.) under video-assisted thoracoscopy (VATS) was the most popular operative technique in PSP treatment due to its low recurrent risks (2–14%) (3,5,6). While recently a lot more evidence have shown that pleurodesis could not lower the recurrence rate of PSP (7). Besides, it might introduce the risk of bleeding and of injury to surrounding structures, which could lead to more postoperative drainage, prolonged in-hospital stay, and extra medical related cost.
This retrospective analysis of historical data from our institution aimed to compare the perioperative data and long-term efficacy between bullectomy with pleural abrasion (BLPA) and bullectomy group.
Methods
The study was approved by institutional ethics committee board of China Japan Friendship Hospital (No. 2015-GZR-74). Written informed consent from each patient was waived as the nature of retrospective study. All PSP patients who underwent VATS bullectomy (120 bullectomy cases) or BLPA (225 BLPA cases) in Department of General Thoracic Surgery, China Japan Friendship Hospital between 2008.1 and 2013.12 were retrospectively reviewed. The criteria were as follows: both first episode and recurrent PSP patients who underwent either bullectomy or BLPA via VATS were included based on British Thoracic Society (BTS) or American College of Chest Physicians (ACCP) guidelines. Patients with underlying pulmonary diseases (chronic obstructive pulmonary disease, tuberculosis, etc.), who did not write the informed consent, or have undergone thoracic operation that might obstruct VATS bullectomy or pleurodesis were excluded.
All operations were performed under general anesthesia with single lung ventilation by double-lumen endotracheal intubation. Two-port VATS technique was regularly adopted. Whether to perform pleural abrasion or not was decided by the patient’s preoperatively after inform consent. In BLPA cases, parietal pleura above the 5th intercostal space were abrased when bullae were found on the surface of the upper lobe. Total parietal pleura were abrased when bullae were found on the surface of other lobe or both upper lobe and other lobe.
Outpatient follow-up were carried out 1 month, 6 months, and 1 year. Telephone follow-up were carried out thereafter. Patients were asked to take chest X-ray during outpatient clinic follow-up or when they got symptoms that might be indication of PSP recurrence (chest pain, dyspnea, etc.). In uncertain cases, chest computed tomography was used to exclude recurrence.
The following data of each patient was recorded: gender, age, smoking history, body mass index (BMI), side of pneumothorax, bullae characteristics (location, number, absence or presence), duration of operation, blood loss, postoperative drainage, length of chest tube removal, morbidity and mortality, total cost and recurrence. Recurrence was defined as chest X-ray or computed tomography confirmed pneumothorax on the operative side in operation patients, with or without possible symptoms.
SPSS for Windows version 20.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Continuous variables are compared using student’s t-test and expressed as the mean and standard deviation (SD). Categorical data are compared with χ2 test or Fisher’s exact test and presented as numbers and percentages. A two-tailed P value less than 0.05 was considered statistically significant. Follow-up analysis was performed using time-to-event data (for which patients were censored at the time of withdrawal from the study or at the last follow-up), with the event rates estimated by Kaplan-Meier methods and compared with the log-rank test. Propensity scores were calculated including preoperative variables: gender, age, smoking history, BMI, side of pneumothorax. Patients data between groups were matched 1:1 and calipered (0.1) based on their propensity scores.
Results
Three hundred and forty five patients (283 men and 62 women) with an average age of 27 (27.32±11.41) years old were included in this study. Sixty three patients were ever smokers, 282 patients were never smokers. Left side pneumothorax occurred in 180 cases, right side pneumothorax occurred in 165 cases. One hundred and twenty bullectomy and 225 BLPA have been performed for included patients.
There were no significant difference in age, sex, smoking history, side of pneumothorax (left or right), and intraoperative blood loss between bullectomy and BLPA groups. There were significant differences in BMI (20.2±3.2 vs. 19.5±2.8, P=0.04), operation duration (88.97±42.27 vs. 68.83±34.71, P<0.01), number of bullae (P<0.01), and upper lobe bullae (P=0.01). There was no mortality or significant complication in both groups. More postoperative drainage (1,170.66±904.02 vs. 528.38±491.49, P<0.01), and longer chest tube removal days (6.59±4.29 vs. 4.76±2.67, P<0.01) were observed in BLPA group. Total medical cost was significantly higher in BLPA group (4,703.86±1,526.31 vs. 4,204.64±1,203.90, P<0.01) (Table 1).
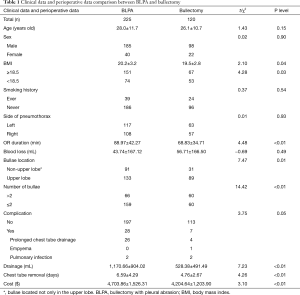
Full table
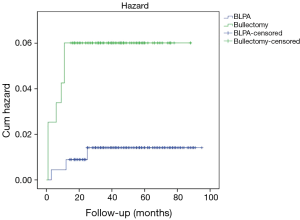
Ten recurrence occurred after a mean follow-up of 47 months (range, 1 to 90 months), nine of which occurred within the first 12 months. One more recurrence occurred 25 months after operation. Two cases resolved by observation, six by drainage, and two received re-operation. Three (3/225, 1.3%) and seven (7/120, 5.8%) recurrence occurred in BLPA and bullectomy groups respectively. Significant difference existed in recurrence rate between BLPA group and Bullectomy group (P=0.02) (Figure 1). Compared with patients with high BMI (≥18.5), patients with low BMI (<18.5) got higher recurrence rate (P=0.03). No significant difference was found in patients’ recurrence with different sex, smoking history, number of bullae, and bullae location.
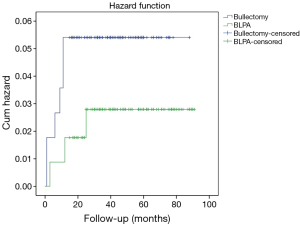
After propensity score match, 114 patients were included in both bullectomy and BLPA groups. As Table 2 illustrated, there was no significant difference in age, sex, BMI level, smoking history, side of pneumothorax (left or right), intraoperative blood loss, and complication rate between BLPA and bullectomy groups. There were significant differences in operation duration (90.50±42.90 vs. 67.88±31.96, P<0.01), bullae location (P=0.02), and number of bullae (P<0.01). There was no mortality or significant complication in both groups. More postoperative drainage (1,280.18±1,071.04 vs. 523.55±484.79, P<0.01), and longer chest tube removal (6.53±4.16 vs. 4.69±2.63, P<0.01) were observed in BLPA group. Total medical cost was significantly higher in BLPA group (4,700.69±1,591.56 vs. 4,211.45±1,207.7, P=0.01). 3 (3/114, 2.6%) and 6 (6/114, 5.3%) recurrence occurred in BLPA and bullectomy groups respectively. There was no significant recurrence difference between BLPA group and bullectomy group (P=0.30) (Figure 2). Also no significant difference was found in patients’ recurrence rate with different BMI level, different sex, smoking history, number of bullae, and bullae location.
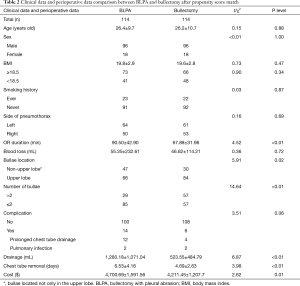
Full table
Discussion
The aim of this retrospective study was to compare the safety, perioperative and long-term efficacy between BLPA and bullectomy group. Our results showed no significant difference in postoperative complication rate between the two groups. Compared with bullectomy group, the BLPA groups were associated with longer operation duration, more postoperative drainage, longer chest tube removal days, and more medical cost both before and after propensity score match. A higher recurrence ratio was firstly observed in bullectomy group, while after propensity score match, this significant difference between BLPA and bullectomy groups disappeared.
Pleurodesis has been widely used in PSP treatment for many years, and its efficacy has been clearly shown by Hatz and colleagues (8). In their study, 109 spontaneous pneumothorax patients who underwent VATS bullectomy with or without pleurodesis were followed up. Pleurodesis were used in 37 patients. Five cases recurred after a median follow-up of 53.2 months, none of which received pleurodesis. Based on these results, they concluded that pleurodesis should be recommended in all spontaneous pneumothorax cases. The importance of pleurodesis has also been clarified by several other authors (3,5). Different pleurodesis techniques such as pleural abrasion, pleurectomy, surgical chemical pleurodesis (instillation of talc or tetracycline during operation, etc.) have been investigated in history. Published evidence have shown that overall success rate of pleurodesis in pneumothorax treatment could be over 90% (9,10). Several studies have reported different outcomes of various pleurodesis techniques (11,12). Based on the above published outcome, VATS bullectomy with pleurodesis was recommended as the most efficient way of preventing recurrence in recurrent PSP patients by the BTS and ACCP guidelines have come to the consensus on management of PSP (2,3).
However, several questions still remained unresolved. The first is how to determine the presence or extent of adhesions produced by pleurodesis. There is no imaging method (computed tomography, ultrasonography, etc.) that could accurately assess the extent of pleural adhesion (13). Besides, adhesions formed by pleurodesis might complicate future thoracic surgery. Recently, the efficiency of pleurodesis in controlling the recurrence of PSP has been challenged by several authors (6,7). In one prospective randomized clinical trial conducted by Min and colleagues (7), 289 patients were enrolled in BLPA and bullectomy group. After a mean follow-up of 18 months, their results showed that recurrence rate was not significantly different between the two groups. However, intraoperative bleeding and postoperative drainage were significantly higher in BLPA group.
Recently a systemic review and meta-analysis also got the similar results (14). In this meta-analysis, the efficiency of BLPA was investigated and compared with bullectomy in PSP patients. After enrolled six randomized clinical trials, their results showed that BLPA did not decrease recurrence rate of pneumothorax compared with wedge resection alone (P=0.791), but intraoperative blood loss and postoperative drainage were higher in BLPA group. They concluded that pleural abrasion should not be recommended routinely after thoracoscopic bullectomy due to the greater incidence of adverse effects.
In our practice, all patients were informed with the potential benefit and defect of pleural abrasion. Then whether to perform pleural abrasion or not was decided by the patient’s preoperatively. Total recurrence rate of 345 patients underwent bullectomy with or without pleural abrasion was 2.9%. Compared with bullectomy, BLPA decreased the recurrence rate of PSP from 5.8% in bullectomy group to 1.3% in BLPA group significantly. After propensity score match, though still higher recurrence rate was observed in bullectomy group (5.3% vs. 2.6%), the significant recurrence difference between the two groups disappeared.
Our results also showed that additional pleural abrasion increased operation duration (90.5 vs. 67.88 min), postoperative pleural drainage (1,280.18 vs. 523.55 mL), and chest tube removal days (6.53 vs. 4.69 days) both before after propensity score match, these results should be caused by the pleuritis that occurred after intraoperative pleural abrasion. The financial burdens were proved to be increased by performing pleural abrasion. An average of $4,700.69 has been spent in BLPA group compared with $4,211.45 in bullectomy group. The increasing operation duration and longer chest tube removal days in BLPA group could be an explanation of this higher financial burden. Our results together with other published studies (7,14), gave further evidence that pleura abrasion should not be routinely used in PSP patients.
There was no postoperative mortality and severe morbidity in our study. However, the total morbidity 10.0% (35/345) was much higher compared with the other studies. Thirty out of 35 were prolonged chest tube removal (>5 days), most of which (26/30) occurred in BLPA group. This might be probably caused by much more postoperative drainage in BLPA group. Postoperative air leak should also be considered as the other explanation.
There are several limitations in this study. First, selection bias might exist as this study was retrospective one and all cases derived from a single facility. Second, we didn’t perform multivariate analysis as the lower recurrent rate (2.9%) of included patients could limit its power. However, the results of our study could be strengthened by propensity score match. This could balance the confounding factors that might influence the recurrence rate between BLPA and bullectomy groups. Still further randomized clinical trial was needed to verify these results. Third, the median follow up in our study is 47 months, long-term follow-up were still needed and may change our results.
Conclusions
This retrospective study showed that compared with bullectomy alone, BLPA could provide similar recurrence rate for PSP patients, but at the price of longer operation time, longer chest tube removal days, and more medical cost. Based on outcome of this study, BLPA should not be routinely performed in PSP cases.
Acknowledgements
Funding: This project was supported by the National Major Clinical Program of China: [2011]873.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee board of China Japan Friendship Hospital (No. 2015-GZR-74). Written informed consent from each patient was waived as the nature of retrospective study.
References
- Melton LJ 3rd, Hepper NG, Offord KP. Melton LJ 3rd, Hepper NG, Offord KP. Am Rev Respir Dis 1979;120:1379-82. [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Luh SP. Review: Diagnosis and treatment of primary spontaneous pneumothorax. J Zhejiang Univ Sci B 2010;11:735-44. [Crossref] [PubMed]
- Chen JS, Hsu HH, Kuo SW, et al. Management of recurrent primary spontaneous pneumothorax after thoracoscopic surgery: should observation, drainage, redo thoracoscopy, or thoracotomy be used? Surg Endosc 2009;23:2438-44. [Crossref] [PubMed]
- Park JS, Han WS, Kim HK, et al. Pleural abrasion for mechanical pleurodesis in surgery for primary spontaneous pneumothorax: is it effective? Surg Laparosc Endosc Percutan Tech 2012;22:62-4. [Crossref] [PubMed]
- Min X, Huang Y, Yang Y, et al. Mechanical pleurodesis does not reduce recurrence of spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:1790-6; discussion 1796.
- Hatz RA, Kaps MF, Meimarakis G, et al. Long-term results after video-assisted thoracoscopic surgery for first-time and recurrent spontaneous pneumothorax. Ann Thorac Surg 2000;70:253-7. [Crossref] [PubMed]
- Kennedy L, Sahn SA. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest 1994;106:1215-22. [Crossref] [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [Crossref] [PubMed]
- Ayed AK, Al-Din HJ. The results of thoracoscopic surgery for primary spontaneous pneumothorax. Chest 2000;118:235-8. [Crossref] [PubMed]
- Chang YC, Chen CW, Huang SH, et al. Modified needlescopic video-assisted thoracic surgery for primary spontaneous pneumothorax: the long-term effects of apical pleurectomy versus pleural abrasion. Surg Endosc 2006;20:757-62. [Crossref] [PubMed]
- Acton V. Is pleurodesis for the treatment of primary spontaneous pneumothorax a misnomer--and if it works, does it matter? J Thorac Cardiovasc Surg 2015;149:397-8. [Crossref] [PubMed]
- Ling ZG, Wu YB, Ming MY, et al. The effect of pleural abrasion on the treatment of primary spontaneous pneumothorax: a systematic review of randomized controlled trials. PLoS One 2015;10:e0127857. [Crossref] [PubMed]


