Primary atypical carcinoid tumor of the mediastinum: a very rare finding
Introduction
Neuroendocrine tumors (NETs) are epithelial neoplasms with predominant neuroendocrine differentiation that arise in most organs of the body (1). Primary NET of the mediastinum are very rare (2); they have been the source of much attention and controversy in the literature because of their origin, that is still debated, and their nomenclature and classification that have evolved over the years.
Clinically, patients may be asymptomatic, or manifest local symptoms due to the compression or invasion of mediastinal structures, or systemic symptoms secondary to the tumor capacity to produce hormones or cytokines (3). Chest CT-scan is important to define the characteristics of the tumor and its anatomical relationships with surrounding structures. Definitive diagnosis is based on histopathological examination and immunophenotypic markers. We present the case of a 50-year-old man with a primary atypical carcinoid tumor of the anterosuperior mediastinum treated by multimodal therapy with excellent result.
Case presentation
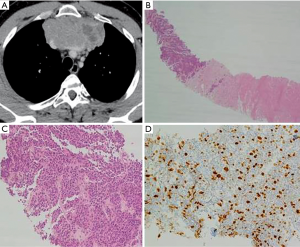
A 50-year-old man, never smoker, with no significant past medical history, presented for asthenia, dyspnea, and substernal sense of weight for two weeks. A chest-CT scan revealed a tumor mass of 107×55×95 mm3 localized in the anterosuperior mediastinum causing the compression of both brachiocephalic veins, superior vena cava, pericardium, and lungs (Figure 1A). A FDG-PET demonstrated high FDG uptake only in the mediastinal mass; no metastases were seen. CT-guided needle biopsy revealed a NET (Figure 1B,C). Tumor cells were immunoreactive for synaptophysin; Ki67 was 20% (Figure 1D). The patient underwent four cycles of neoadjuvant chemotherapy with cisplatin and gemcitabine. A new chest CT-scan showed a significant reduction of the tumor mass (54×56×80 mm3) (Figure 2A). Then, a surgical excision was proposed through a median sternotomy. On exploration, the left anonymous vein was almost totally invaded by the tumor; both lungs were marginally infiltrated. Firstly, a double pulmonary wedge resection was performed. Secondly, the left anonymous vein was dissected and sectioned. Then, an invasion of the right anonymous vein at the confluence with the superior vena cava was observed after partial pericardiectomy. So, under total caval clamping, a partial resection of the vena cava and reconstruction with a bovine pericardium patch (Figure 2B) was carried out (clamping time: 27 minutes). The postoperative course was uneventful. Seven days later the patient was discharged home. Although the resection margins were negative, in view of the fact that the tumor was found to infiltrate different organs (lung, vessels), after multidisciplinary discussion the patient underwent 25 sessions of adjuvant radiotherapy (total dose 55 Gy). Fifty-one months after surgery, he is doing well and free of disease.
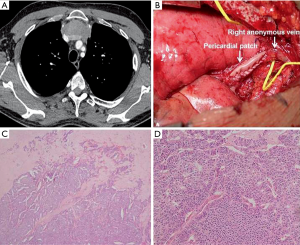
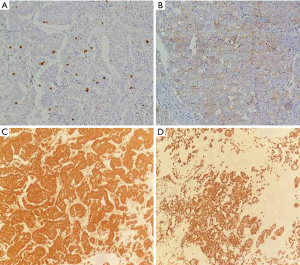
Macroscopically the mass measured 80×30×90 mm3; the cut surface was yellowish-white. The specimen was entirely sampled. Microscopically it showed clusters of medium-sized cells, sometimes with cuboidal morphology, eosinophilic cytoplasm with nuclear pleomorphism and small nucleoli, arranged in trabeculae, nests and lobules and immersed in a hyaline stroma with calcifications and areas of necrosis (Figure 2C,D). The tumor surrounded medium-sized vessels with perineural and vascular invasion. An infiltration of the adjacent lung parenchyma was observed. No thymic tissue was found. The Ki67 was 10% (Figure 3A). Immunohistochemically the tumor cells were positive for CD117 (Figure 3B), synaptophysin (Figure 3C), chromogranin (Figure 3D), CD56 [neural cell adhesion molecule (NCAM)], cytokeratin pool, [epithelial membrane antigen (EMA)], and negative for thyroid transcription factor-1 (TTF1), leukocyte common antigen (LCA), fibroblast-activation protein (FAP), prostate-specific antigen (PSA), P63, CD5 and CD99. Mitoses were found to be 8/10 HPF (according to WHO 2015). The somatostatin receptor R2A (SSTR2A) score was 0. Considering the radiological and morphological appearance, as well as the immunophenotypic characteristics, a diagnosis of primary NET of the mediastinum, intermediate grade (G2), atypical carcinoid according to WHO 2015, was formulated.
Discussion
NETs may arise in any organs and tissues. They are more frequently localized in the gastro-entero-hepatic or respiratory system (4,5). NETs of the mediastinum are very rare; among these, thymic NETs are the most frequent, they are exclusively located in the anterosuperior mediastinum and account for 2% to 4% of all mediastinal tumors (2).
Although mediastinal NETs occur more frequently in the anterior mediastinum, rare cases in the middle (6) and posterior mediastinum have been reported in the literature. Mediastinal NETs have been the source of much attention and controversy among scientists and researchers because of their origin, that is still debated, and their nomenclature and classification that have evolved over the years (2).
They may originate from neuroendocrine elements within the thymus (most frequently), from aorticopulmonary and paravertebral paragangliomas (paraganglionic rests), from misplaced embryonal structures within the mediastinum and even from ectopic or supernumerary parathyroid glands (2,7,8). In the literature, sporadic cases of NETs arising from a mediastinal teratoma have also been described (9).
We describe the case of a primary atypical carcinoid tumor of the anterosuperior mediastinum. The differential diagnosis could include an adenocarcinoma, small cell carcinoma (SCC) and neuroendocrine SCC of the thymus, paraganglioma, neuroblastoma, peripheral primitive neuroectodermal tumor (PNET), rhabdomyosarcoma, malignant lymphoma and mediastinal primordial germ cell tumor.
A diagnosis of adenocarcinoma was excluded because of the presence of dense-cored neurosecretory granules, the absence of bronchial tissue within the tumor and the immunohistochemical negativity for TTF-1. Thinking about a SSC, the cytoplasm of our tumor cells was larger than those usually seen in SCC. Moreover, the presence of nucleoli and a Ki67 proliferative index ranging from 10% to 20% (after and before chemotherapy, respectively) exclude the diagnosis of SCC. The immunohistochemical positivity for epithelial markers, including cytokeratin, and EMA allowed us to rule out the possibility to face a paraganglioma or neuroblastoma. With regard to peripheral primitive neuroectodermal tumors, they may develop in the thoraco-pulmonary paravertebral portion and are characterized by a solid organoid growth of monomorphic cells with ill-defined borders and they commonly exhibit several epithelial markers, including cytokeratin, and EMA. The tumor cells observed in our patient, varied in size and pleomorphism more than those characterizing PNET; the cytoplasm was also eosinophilic with some prominent nucleoli. Furthermore, the tumor tissue contained only Flexner-Wintersteiner rosette-like glands, while PNET usually contained only Homer-Wright-type rosettes. Lastly, tumor cells were negative for CD99 (MIC2) and did not contain PAS positive granules, while PNET expressed positivity for CD99 in most of the cases.
The positivity for epithelial markers puts aside the diagnosis of rhabdomyosarcoma; and the negativity for lymphoid markers excludes a lymphoid lesion. Since germ cells tumor markers were negative, we could hypothesize that the origin of this tumor was not from mediastinal primordial germ cells. It is very important to note that we could not identify any thymic residual in our specimen, such as areas of lymphoid hyperplasia or Hassall’s corpuscles. Moreover, thymic markers such as P63 and CD5 were negative at immunohistochemistry.
Many authors argued that a co-expression of CD117 (c-kit) and CD5 could be suggestive of a neoplasm of thymic origin (9,10). In our case only CD117 was positive, therefore not suggesting a thymic origin. CD117 is usually positive in small cell lung carcinoma and large cell NET and it is usually negative in typical and atypical carcinoid. The positivity observed for c-kit could have important therapeutic implication, such as the administration of imatinib—a tyrosine-kinase inhibitor—in case of disease recurrence (11).
Compared with bronchopulmonary carcinoid, primary NETs of the mediastinum are characterized by a poorer prognosis due to their high propensity for local recurrence and earlier distant metastases (12). The role of chemotherapy is debated; in this case chemotherapy was effective, having determined a reduction of tumor size and a decrease of proliferation rate. In the literature, some cases of mediastinal NETs successfully treated by a combination of chemotherapy and Y-DOTATOC (13) or by radiotherapy alone (14) have been described. Somatostatin-analogue therapy could represent a good option in non-operable patients or in case of systemic recurrence in SSTRs-positive NETs. Most of non-pulmonary NETs arising in the mediastinum are of thymic origin. In the present case, no evidence of thymus involvement and no thymic tissue remnants were identified within the lesion. Considering all the above mentioned characteristics, including the absence of thymic tissue, this tumor could be defined as a primary NET of the mediastinum of intermediate grade (atypical carcinoid according to WHO 2015). The present case demonstrated the difficulties that could be encountered when applying the current criteria for classifying the NETs of the mediastinum (15). Mediastinal NETs are usually very aggressive but our patient demonstrates that even in case of locally advanced disease, multimodal treatment could reach a radical intent, with the possibility of a long-term survival. Further studies are necessary to elucidate the histogenesis and origin of these neoplasms.
Acknowledgements
None.
Footnote
Conflicts of interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [Crossref] [PubMed]
- Suster S, Moran CA. Neuroendocrine neoplasms of the mediastinum. Am J Clin Pathol 2001;115 Suppl:S17-27. [PubMed]
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Caplin ME, Baudin E, Ferolla P, et al. Ann Oncol 2015;26:1604-20. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Evangelista A, et al. Prognostic model of survival for typical bronchial carcinoid tumours: analysis of 1109 patients on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours Working Group. Eur J Cardiothorac Surg 2015;48:441-7; discussion 447. [Crossref] [PubMed]
- Pikin O, Sidorov D, Amiraliev A, et al. Giant neuroendocrine tumour localized to the thoracoabdomen. Eur J Cardiothorac Surg 2013;44:385. [Crossref] [PubMed]
- Maeda A, Nakata M, Yasuda K, et al. Unknown primary large cell neuroendocrine carcinoma (LCNEC) in the mediastinum. Gen Thorac Cardiovasc Surg 2013;61:542-5. [Crossref] [PubMed]
- Kacar F, Meteoglu I, Sen S, et al. Primary neuroendocrine carcinoma of the mediastinum. Pathol Oncol Res 2002;8:200-1. [Crossref] [PubMed]
- Schaefer IM, Zardo P, Freermann S, et al. Neuroendocrine carcinoma in a mediastinal teratoma as a rare variant of somatic-type malignancy. Virchows Arch 2013;463:731-5. [Crossref] [PubMed]
- Kriegsmann M, Muley T, Harms A, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: a large scale study of 1465 non-small cell lung cancer cases. Diagn Pathol 2015;10:210. [Crossref] [PubMed]
- Butnor KJ, Burchette JL, Sporn TA, et al. The spectrum of Kit (CD117) immunoreactivity in lung and pleural tumors: a study of 96 cases using a single-source antibody with a review of the literature. Arch Pathol Lab Med 2004;128:538-43. [PubMed]
- Gal AA, Kornstein MJ, Cohen C, et al. Neuroendocrine tumors of the thymus: a clinicopathological and prognostic study. Ann Thorac Surg 2001;72:1179-82. [Crossref] [PubMed]
- Fazio N, Grana C, Pelosi G, et al. Successful chemotherapy and 90Y-DOTATOC in a patient with mediastinal highly aggressive neuroendocrine carcinoma. Acta Oncol 2006;45:627-9. [Crossref] [PubMed]
- Furuta M, Hayakawa K, Kato S, et al. Malignant neuroendocrine tumor presenting a huge mediastinal mass controlled with radiation therapy. Lung Cancer 1998;22:55-8. [Crossref] [PubMed]
- Moran CA. Primary neuroendocrine carcinomas of the mediastinum: review of current criteria for histopathologic diagnosis and classification. Semin Diagn Pathol 2005;22:223-9. [Crossref] [PubMed]


