Standard imaging techniques in transcatheter aortic valve replacement
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as the preferred treatment strategy in patients with severe, symptomatic aortic stenosis considered at intermediate, high or extreme risk for surgical aortic valve replacement. Its use may soon be extended to patients at low risk, for surgery as well (1-4). While TAVR offers the benefit of a minimally invasive or entirely percutaneous approach with more rapid recovery, the preoperative evaluation entails extensive imaging utilizing a variety of modalities. While this complex work-up is necessary to ensure optimal outcomes, it requires not only the technology but the expertise in interpretation to extract the maximal benefit from the information obtained.
Valve prostheses
Two valve prostheses are currently available and Food and Drug Administration (FDA) approved in the United States for TAVR: the self-expandable Medtronic CoreValve (Medtronic, Inc., Minneapolis, MN, USA) and the balloon-expandable Edwards Sapien Valve (Edwards Lifesciences, Irvine CA, USA).
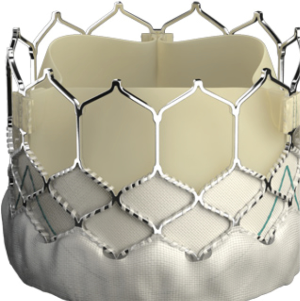
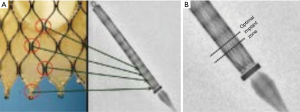
The landmark Partner trials utilized the Edwards Sapien valve made of bovine pericardium in a stainless steel frame. It was available in 23 and 26 mm sizes and was delivered via a 22–24F sheath. The next generation Sapien XT valve utilized bovine pericardium in a cobalt chromium frame, and came in 23, 26, and 29 mm sizes requiring a 16–20F sheath. The latest version, the Edwards Sapien III, has an outer skirt designed to decrease paravalvular aortic insufficiency (AI) (Figure 1). It is available in 20, 23, 26, and 29 mm sizes and utilizes a 14-16F sheath (Table 1).
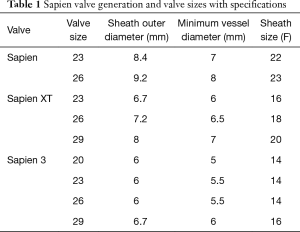
Full table
The Medtronic CoreValve utilizes bovine pericardium in a nitinol frame (Figure 2). Nitinol is unique in that it demonstrates superelastic properties at low temperatures allowing for tight compression on a small sheath, but generates very high radial forces at higher body temperatures. This allows for valve deployment without a balloon (5). The latest version of the Medtronic CoreValve, the Evolut R, utilizes porcine pericardium and has a recapturable feature and an extended skirt designed to decrease malpositioning and rates of postoperative AI. Both come in a variety of sizes [23 mm (Evolut R), 26 mm (Evolut R), 29 mm (Evolut R), 31 mm (CoreValve), 34 mm (Evolut R)], and the Evolut R is delivered via a 14-16F sheath (Table 2).
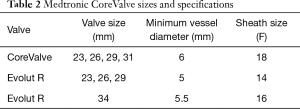
Full table
Preoperative evaluation
Transthoracic echocardiography (TTE) remains the mainstay of diagnosis of aortic stenosis. The transaortic gradient, velocity and aortic valve area are all determined reliably with TTE. TTE can also assess the degree of valve calcification and valve morphology (bicuspid versus tricuspid) which is highly relevant given that TAVR is still controversial in bicuspid aortic stenosis (6-8). TTE can assess ventricular function and other valvular and structural abnormalities, such as septal hypertrophy which in severe cases may also be a contraindication as it is a risk for valve embolization (9). Ascending and descending aortic atheroma can also be visualized and, if severe, may affect access choice. TTE is readily available, entails no patient discomfort, and is risk free. However, it is highly user dependent and in certain patient populations, such as the obese, those with severe emphysematous lung disease, and those with prior thoracic surgery, imaging may be suboptimal.
In addition to diagnosing and quantifying the degree of aortic stenosis, the TAVR preoperative evaluation entails sizing of the annulus and surrounding structures to determine the optimal prosthesis type and size and to determine the adequacy of the peripheral vasculature for selection of an access strategy. Computed tomographic angiography (CTA) has become the modality of choice for annular sizing with low interobserver variability (10,11) and reductions in postoperative AI and valve related complications (12-14) when compared to echocardiography guided TAVR demonstrated in several studies.
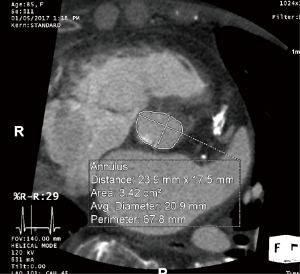
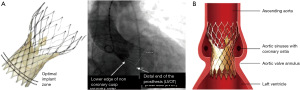
The aortic annulus is a virtual ring formed by the nadirs of the aortic leaflets, and assessment of its dimension is the most critical aspect of imaging in the preoperative TAVR evaluation. As most annuli are elliptical, measurements of the major and minor diameters, annular area, and annular perimeter are taken. The area- and perimeter-based diameters as well as the average of the major and minor diameters are then determined (Figure 3). Undersizing of the prosthetic valve may lead to paravalvular AI and embolization, whereas oversizing may lead to annular rupture and coronary occlusion. Measurements are preferably taken during systole (20–40% R-R interval) with EKG-triggered cardiac CTA as this is when the diameter is largest, although this is controversial (5,15,16).
TTE and transesophageal echocardiography (TEE) can and have been used for determination of annular dimensions, although they frequently result in underestimation due to the assumption of a circular annulus (17-19). Annular measurements by cardiac CTA have resulted in lower rates of paravalvular AI, a major determinant of short- and long-term outcomes (13,14,20). Measurements of annular perimeter tend to yield the lower interobserver variability (10) and larger diameters than annular area or the mean of the major and minor diameters (16,21). However, a larger measured diameter necessitates a larger prosthesis, with the risk of annular rupture. Given that the incidence of annular rupture with the self-expandable CoreValve is nearly nonexistent, perimeter-based measurements are frequently used to reduce the rate of paravalvular AI, whereas the balloon-expandable Edwards Sapien system uses area-based measurements.
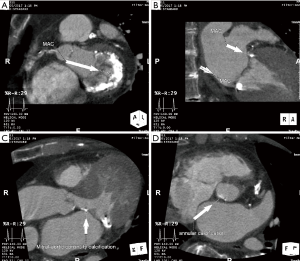
Cardiac CTA is also used to determine the dimensions of the sinuses of Valsalva and sinotubular junction, and the height of the coronary ostia and the sinotubular junction (Figures 4). TAVR entails implantation of a prosthesis in the aortic annulus with lateral displacement of the native leaflets and associated calcium. Coronary obstruction is a rare complication of TAVR caused by displacement of leaflet or annular calcium by the prosthesis into the coronary ostia and is more common with balloon-expandable valves (22). Smaller sinus of Valsalva and sinotubular junction dimensions and coronary ostia height <10 mm are risk factors for coronary obstruction, and may warrant coronary wire access prior to valve deployment (5,16,22) (Tables 3,4).
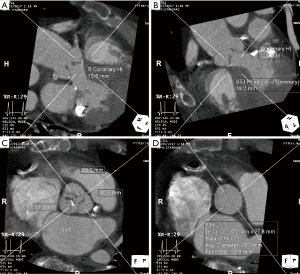

Full table

Full table
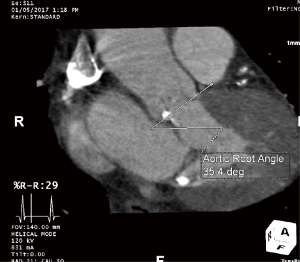
Cardiac CTA can assess the degree of annular, leaflet, and left ventricular outflow tract calcification. Excessive leaflet calcification is a potential risk for coronary obstruction, and excessive left ventricular outflow tract or annular calcification is a risk factor for paravalvular AI (especially asymmetric calcification) and annular rupture (16) (Figure 5). Cardiac CTA can measure the angle between the left ventricular outflow tract and the aorta. There is evidence to suggest that patients with a high degree of aortic angulation may have increased rates of postoperative AI and other valve related complications with self-expandable but not balloon-expandable valves (23-25), and accordingly, patients with aortic angulation greater than 70 degrees were excluded from trials of the Medtronic CoreValve (Figure 6). Finally, cardiac CTA can assess left ventricular function and other valvular abnormalities, similar to echocardiography, and coronary artery disease.
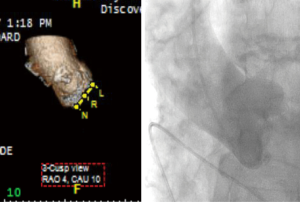
At the time of valve deployment during TAVR, the correct fluoroscopic view in which all three cusps are seen in line is obtained. This deployment angle requires multiple “trial-and-error” aortograms with repositioning of the fluoroscopy arm until an adequate 3-cusp view is seen, but can be predicted with cardiac CTA. Cardiac CTA guided determination of the deployment angle may reduce procedure time, contrast volume, and radiation exposure, with improvements in the rate of correct fluoroscopic projections at the time of TAVR and improved outcomes (16,20,26) (Figure 7).
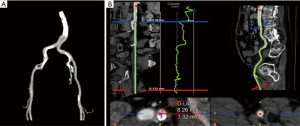
The transfemoral route is the preferred route for TAVR access, with reductions in patient discomfort, pulmonary complications, and length of stay compared to other approaches. However, in the setting of inadequate peripheral vasculature, vascular complications can be catastrophic, and alternative approaches including subclavian, transaortic, transapical, and transcarotid access are utilized (27-31). Peripheral CTA is used to determine the adequacy of the peripheral vasculature, with assessment of femoral and iliac artery dimensions, tortuosity, and degree of calcification (Figures 8). Other abnormalities, such as significant atheroma or dissection are also noted. The newest iterations of currently available devices are delivered with 14- or 16-F sheaths and require 5 or 5.5 mm access vessels at a minimum. Circumferential or horseshoe calcification or tortuosity with greater than 90 degrees angulation are contraindications for peripheral access as well.
In cases in which CTA is contraindicated, as in patients with renal insufficiency, TEE may be used for annular sizing. Three-dimensional TEE can measure several of the parameters assessed with cardiac CTA, although due to the scalloped nature of the aortic annulus, evidence suggests that TEE may undersize it relative to CTA, and thus CTA is the preferred modality (14,17-19). CTA is mandatory to evaluate the peripheral vasculature, and in the setting of renal insufficiency, low dose contrast administration via a catheter placed in the abdominal aorta may allow for adequate assessment of the peripheral vessels and combined with TEE for annular measurements. Finally, extra-cardiac findings may be noted on CTA that represent contraindications for TAVR. Such findings are present on almost a quarter of scans done for TAVR evaluation, with lesions suspicious for malignancy being the most common, although a clear association with decreased long term survival has not been demonstr (16,32).
Intraoperative evaluation
In most centers, TAVR is performed under general anesthesia with fluoroscopy and TEE. TEE is used to confirm the degree of aortic stenosis, valve morphology, ventricular function, and assess for valvular or other abnormalities.
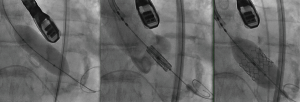
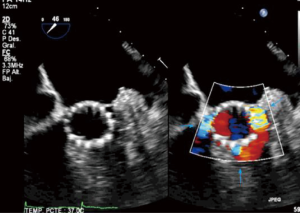
In standard transfemoral approaches, under fluoroscopic guidance, a pacing wire is placed in the right ventricle and a pigtail catheter is placed in the noncoronary sinus. An aortogram is performed and the deployment angle which yields the three-cusp view determined. As described earlier, this can be predicted from cardiac CTA. The aortic valve is then crossed with a stiff wire under fluoroscopic guidance and entanglement with the mitral subvalvular apparatus ruled out by TEE. Balloon valvuloplasty is performed if necessary. The valve is then placed across the annulus and appropriate positioning confirmed by fluoroscopy and TEE. The valve is deployed (Figure 9) and paravalvular AI assessed. This is best done by TEE, although more severe degrees of AI can be noted on fluoroscopy. On echocardiography, an AI jet occupying >20% of the circumference of the prosthesis on short-axis view is considered severe (Figure 10). Additional balloon valvuloplasty is performed if needed under fluoroscopic guidance. TEE is used to assess postoperative valve function, including leaflet motion, valve gradients, and ventricular function. All catheters and wires are then removed. Fluoroscopy can again determine the patency of the coronary and access vessels if there is any question.
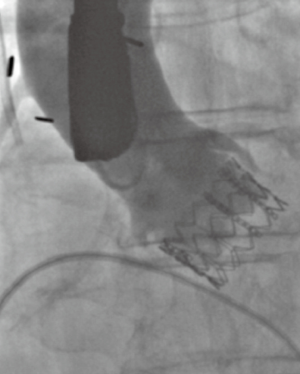
The Edwards Sapien III valve is ideally deployed with approximately 20–30% of the stent frame on the ventricular side, and 70–80% on the aortic side (Figure 11). This prosthesis is thus placed in an intra-annular position. The Medtronic CoreValve, when collapsed in its sheath, displays a series of lines on fluoroscopy that correspond to the intersections of the nitinol lattice (Figure 12). These markers are used to deploy the valve ideally with only 4–6 mm of the stent frame on the ventricular side (Figure 13A). The valve leaflets are thus on a supra-annular place (Figure 13B), which is beneficial with regards to a larger effective orifice area, especially in the setting of valve-in-valve procedures (Figure 14).
New wall motion abnormalities may suggest coronary occlusion, requiring coronary angiography and stenting or surgery. In the setting of low coronary ostia, coronary access may be obtained prior to valve deployment to assist in a coronary intervention if needed after valve deployment. Significant AI may suggest a malpositioned valve, which may require deployment of a second valve. A new pericardial effusion may indicate right or left ventricular perforation by the pacing wire or stiff wire, or may indicate annular rupture. Milder cases may be treated by reversal of anticoagulation and observation or pericardiocentesis, although annular rupture will frequently require surgical intervention. New or worsened mitral regurgitation may indicate mechanical injury to the mitral subvalvular apparatus or low prosthesis implantation depth with impingement on the anterior mitral leaflet.
In centers with extensive experience, TAVR with local anesthesia and sedation is more commonly performed. Benefits include avoidance of hemodynamic fluctuations associated with induction of anesthesia, more rapid recovery and shorter length of stay, especially in older patients with extensive pulmonary disease. The downside is that TEE must usually be replaced by TTE. While fluoroscopy is adequate for guidance for the majority of the procedure, AI is better evaluated with TEE, especially milder degrees of AI which still correlate with longer term mortality. TTE is frequently adequate for AI estimation, although in the abovementioned patient populations, such as the obese, those with severe emphysematous lung disease, and those with prior thoracic surgery, imaging may be suboptimal. In addition, while TTE may not interfere with the sterile field in cases performed with transfemoral access, it may be difficult or impossible in alternative access approaches such as transaortic or transapical unless performed with a sterile probe cover. Finally, in the unfortunate setting of a major complication, intubation with general anesthesia will usually need to be performed emergently (33).
Postoperative evaluation
A predischarge and 1-month TTE is generally performed to assess for valve function, including the presence of AI, and assessment of gradients and leaflet motion. Paravalvular AI is not uncommon after TAVR, occurring in 50–85% of patients. AI, even in mild degrees has been associated with increased short and long term mortality. AI of moderate or greater degrees may require reintervention. There is evidence that regurgitant fraction as assessed by cardiac magnetic resonance imaging has a greater association with post-TAVR clinical events than echocardiography (34). Increased leaflet thickness associated with decreased mobility suggests thrombosis. An increase in mean gradient of >10 mmHg may be also noted and may be asymptomatic, or present with symptoms of heart failure. Cardiac CTA is helpful in this scenario to better image the valve leaflets. Anticoagulation is the treatment of choice, with frequent resolution of symptoms and return of gradients to normal (35,36). Follow-up TTE is then obtained at 6 months, 1 year, and yearly thereafter.
Acknowledgements
None.
Footnote
Conflicts of Interest: A Salemi serves as clinical proctor for Edwards Lifesciences, and Medtronic, Inc. BM Worku has no conflicts of interest to declare.
References
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81. [Crossref] [PubMed]
- Wichmann JL, Varga-Szemes A, Suranyi P, et al. Transcatheter Aortic Valve Replacement: Imaging Techniques for Aortic Root Sizing. J Thorac Imaging 2015;30:349-58. [Crossref] [PubMed]
- Mylotte D, Lefevre T, Søndergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014;64:2330-9. [Crossref] [PubMed]
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid Aortic Valve Stenosis: Favorable Early Outcomes With a Next-Generation Transcatheter Heart Valve in a Multicenter Study. JACC Cardiovasc Interv 2016;9:817-24. [Crossref] [PubMed]
- Yoon SH, Lefèvre T, Ahn JM, et al. Transcatheter Aortic Valve Replacement With Early- and New-Generation Devices in Bicuspid Aortic Valve Stenosis. J Am Coll Cardiol 2016;68:1195-205. [Crossref] [PubMed]
- de Biasi AR, Worku B, Skubas NJ, et al. Sigmoid Septum and Balloon-Expandable Transcatheter Aortic Valve Replacement: A Cautionary Tale. J Heart Valve Dis 2015;24:465-7. [PubMed]
- Schmidkonz C, Marwan M, Klinghammer L, et al. Interobserver variability of CT angiography for evaluation of aortic annulus dimensions prior to transcatheter aortic valve implantation (TAVI). Eur J Radiol 2014;83:1672-8. [Crossref] [PubMed]
- Schuhbaeck A, Achenbach S, Pflederer T, et al. Reproducibility of aortic annulus measurements by computed tomography. Eur Radiol 2014;24:1878-88. [Crossref] [PubMed]
- Binder RK, Webb JG, Willson AB, et al. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J Am Coll Cardiol 2013;62:431-8. [Crossref] [PubMed]
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585-95. [Crossref] [PubMed]
- Jilaihawi H, Kashif M, Fontana G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275-86. [Crossref] [PubMed]
- Blanke P, Russe M, Leipsic J, et al. Conformational pulsatile changes of the aortic annulus: impact on prosthesis sizing by computed tomography for transcatheter aortic valve replacement. JACC Cardiovasc Interv 2012;5:984-94. [Crossref] [PubMed]
- Al-Najafi S, Sanchez F, Lerakis S. The Crucial Role of Cardiac Imaging in Transcatheter Aortic Valve Replacement (TAVR): Pre- and Post-procedural Assessment. Curr Treat Options Cardiovasc Med 2016;18:70. [Crossref] [PubMed]
- Vaquerizo B, Spaziano M, Alali J, et al. Three-dimensional echocardiography vs. computed tomography for transcatheter aortic valve replacement sizing. Eur Heart J Cardiovasc Imaging 2016;17:15-23. [PubMed]
- Tsuneyoshi H, Komiya T, Shimamoto T. Accuracy of Aortic Annulus Diameter Measurement: Comparison of Multi-Detector CT, Two- and Three-Dimensional Echocardiography. J Card Surg 2016;31:18-22. [Crossref] [PubMed]
- Husser O, Holzamer A, Resch M, et al. Prosthesis sizing for transcatheter aortic valve implantation--comparison of three dimensional transesophageal echocardiography with multislice computed tomography. Int J Cardiol 2013;168:3431-8. [Crossref] [PubMed]
- Binder RK, Leipsic J, Wood D, et al. Prediction of optimal deployment projection for transcatheter aortic valve replacement: angiographic 3-dimensional reconstruction of the aortic root versus multidetector computed tomography. Circ Cardiovasc Interv 2012;5:247-52. [Crossref] [PubMed]
- Mylotte D, Dorfmeister M, Elhmidi Y, et al. Erroneous measurement of the aortic annular diameter using 2-dimensional echocardiography resulting in inappropriate CoreValve size selection: a retrospective comparison with multislice computed tomography. JACC Cardiovasc Interv 2014;7:652-61. [Crossref] [PubMed]
- Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552-62. [Crossref] [PubMed]
- Abramowitz Y, Maeno Y, Chakravarty T, et al. Aortic Angulation Attenuates Procedural Success Following Self-Expandable But Not Balloon-Expandable TAVR. JACC Cardiovasc Imaging 2016;9:964-72. [Crossref] [PubMed]
- Popma JJ, Reardon MJ, Yakubov SJ, et al. Safety and Efficacy of Self-Expanding TAVR in Patients With Aortoventricular Angulation. JACC Cardiovasc Imaging 2016;9:973-81. [Crossref] [PubMed]
- Sherif MA, Abdel-Wahab M, Stöcker B, et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J Am Coll Cardiol 2010;56:1623-9. [Crossref] [PubMed]
- Gurvitch R, Wood DA, Leipsic J, et al. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc Interv 2010;3:1157-65. [Crossref] [PubMed]
- Storz C, Geisler T, Notohamiprodjo M, et al. Role of Imaging in Transcatheter Aortic Valve Replacement. Curr Treat Options Cardiovasc Med 2016;18:59. [Crossref] [PubMed]
- Arai T, Romano M, Lefèvre T, et al. Direct Comparison of Feasibility and Safety of Transfemoral Versus Transaortic Versus Transapical Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2016;9:2320-5. [Crossref] [PubMed]
- Mylotte D, Sudre A, Teiger E, et al. Transcarotid Transcatheter Aortic Valve Replacement: Feasibility and Safety. JACC Cardiovasc Interv 2016;9:472-80. [Crossref] [PubMed]
- Debry N, Delhaye C, Azmoun A, et al. Transcarotid Transcatheter Aortic Valve Replacement: General or Local Anesthesia. JACC Cardiovasc Interv 2016;9:2113-20. [Crossref] [PubMed]
- Bapat V, Frank D, Cocchieri R, et al. Transcatheter Aortic Valve Replacement Using Transaortic Access: Experience From the Multicenter, Multinational, Prospective ROUTE Registry. JACC Cardiovasc Interv 2016;9:1815-22. [Crossref] [PubMed]
- Stachon P, Kaier K, Milde S, et al. Two-year survival of patients screened for transcatheter aortic valve replacement with potentially malignant incidental findings in initial body computed tomography. Eur Heart J Cardiovasc Imaging 2015;16:731-7. [Crossref] [PubMed]
- Kronzon I, Jelnin V, Ruiz CE, et al. Optimal imaging for guiding TAVR: transesophageal or transthoracic echocardiography, or just fluoroscopy? JACC Cardiovasc Imaging 2015;8:361-70. [Crossref] [PubMed]
- Ribeiro HB, Orwat S, Hayek SS, et al. Cardiovascular Magnetic Resonance to Evaluate Aortic Regurgitation After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;68:577-85. [Crossref] [PubMed]
- Hansson NC, Grove EL, Andersen HR, et al. Transcatheter Aortic Valve Thrombosis: Incidence, Predisposing Factors, and Clinical Implications. J Am Coll Cardiol 2016;68:2059-69. [Crossref] [PubMed]
- Dangas GD, Weitz JI, Giustino G, et al. Prosthetic Heart Valve Thrombosis. J Am Coll Cardiol 2016;68:2670-89. [Crossref] [PubMed]