Elevated serum levels of immunoglobulin A correlate with the possibility of readmission in patients with microscopic polyangiitis
Introduction
Microscopic polyangiitis (MPA) is a kind of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV). ANCA directed against myeloperoxidase (MPO) is found predominantly in patients with MPA and eosinophilic granulomatosis with polyangiitis (1). Most patients first complain about dry cough and dyspnea, and also experience renal insufficiency. In some patients, fever develops and the disease is difficult to diagnose for a long time. Because of the respiratory symptoms of patients with MPA, many cases were initially diagnosed as idiopathic pulmonary fibrosis or interstitial pneumonia on the basis of computed tomography (CT) scan findings (2).
The current treatment regimens for patients with MPA are glucocorticoids and/or immunosuppressants. These regimens are largely successful in controlling AAV; however, in approximately one-fourth of patients, active disease persists or recurs in the first 6 months despite treatment (3). Recently, the monoclonal antibody rituximab was approved for the treatment of MPA, providing the first major alternative to cyclophosphamide for the induction therapy of AAV (4-6). Additionally, a study on 92 patients with AAV showed the clinical benefit of intravenous immunoglobulin (IVIg) as an adjunctive therapy with an acceptable tolerance profile, supporting its use in patients with AAV with relapsing disease (7). Treatment of nonsevere relapses of AAV with an increase in glucocorticoids is effective in restoring temporary remission in most patients; however, recurrent relapses within a relatively short interval remain common. Alternative treatment approaches are needed for this important subset of patients (8,9).
Many recent studies focused on the risk factors of outcomes in patients with MPA. These risk factors included renal insufficiency, lung involvement, diffuse pulmonary hemorrhage (10), any kind of infection, and venous thrombosis (11). The main causes of death within the first year were infection (48%) and active vasculitis (19%). Patients with AAV treated with conventional regimens are at an increased risk of death compared with an age- and sex-matched population (12). In another study, patients with MPA were divided into two pairs of groups: (I) a relapse group and a nonrelapse group according to whether there was recurrence or new onset of disease activity (13); and (II) an infection group and a non-infection group. The results showed that the albumin levels were significantly different between the relapse group and the nonrelapse group. Immunoglobulin G (IgG) levels were identified as factors associated with infectious complications; however, there were no differences in renal prognosis or life prognosis between the two groups (14). Another study also showed that the overall survival rates at 6 and 12 months after a diagnosis of MPA with renal involvement were 79.5% and 71.1%, respectively. Moreover, the severity of renal insufficiency was not related to the survival rate of patients with MPA with renal involvement (15).
In this study, we recruited 57 patients with MPA from our hospital and analyzed the clinical characteristics of these patients. We also evaluated the risk factors for the possibility of readmission in these patients.
Methods
Patients
This is a retrospective study. We recruited 57 patients who met the diagnosis criteria for MPA, as defined by the International Chapel Hill Consensus in 2012, from August 2009 to August 2015 at Beijing Chao-yang Hospital (1). Informed consent was obtained from all patients or their family members. Cases that were not initially diagnosed at our hospital were excluded from this study. The ethics committee of our hospital approved the study protocol.
Data collection
We reviewed the medical records, radiological images, laboratory results, and biopsy findings of the patients. The patients’ age; sex; days of initial hospitalization; number of hospitalizations; symptoms; physical examination, laboratory, and radiological findings; biopsy results; and treatment were all recorded. Readmission was defined as admission at our hospital within 6 months after the first diagnosis and treatment of MPA.
Statistical analysis
Nonnormally distributed variables are summarized as medians, and the two groups were compared by using the Mann-Whitney U-test. Categorical variables are presented as percentages, and the groups were compared by using the chi-square test. Logistic regression analysis was used to screen for risk factors or protective factors. To obtain the cutoff point of variables, receiver operating characteristic (ROC) curves were generated and the area under the curve (AUC) was calculated. Pearson correlation analysis was used to calculate the correlation confidence of two variables. P<0.05 was considered to indicate statistical significance. SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Clinical manifestations
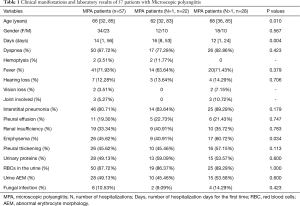
A total of 57 patients with MPA were recruited into this study. The clinical manifestations are summarized in Table 1. This study group comprised 34 male and 23 female patients. The median age of these patients was 66 years (range, 32–85 years). The median number of days of initial hospitalization was 14 days (range, 1–56 days). Respiratory symptoms seemed to be the initial symptoms in many patients, including cough, dyspnea (87.72%), and hemoptysis (3.51%). Systemic symptoms included fever (71.93%), hearing loss (12.28%), vision loss (3.51%), and joint involvement (5.27%). As shown in these patients, respiratory symptoms seemed to be the initial symptoms in most of them.
Full table
Laboratory results
Regular tests
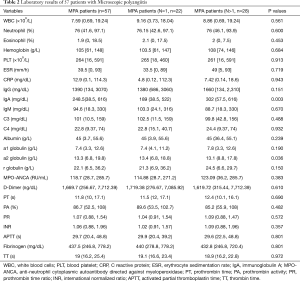
After admission to our hospital, all patients underwent urinalysis; biochemical tests (including tests for liver function, renal function, and electrolyte levels) and D-dimer and coagulation tests (Table 2). The median count of white blood cells was 7.59 109/L (range, 0.69×109/L to 19.24×109/L), and the median proportion of neutrophil and eosinophil was 76% and 1.9%, respectively. The peripheral blood platelet counts ranged from 16 109/L to 148 109/L. Among the 57 patients, 50 (87.72%) had red blood cells in urine showing abnormal erythrocyte morphology. The amount of urine proteins also increased in 28 of these 57 patients (49.13%), and 19 (33.34%) of them developed renal insufficiency. The D-dimer levels of 43 patients with MPA (75.44%) were >500 ng/dL, and only three patients had venous thrombosis according to the ultrasound results. The coagulation tests were normal in most of the patients.
Full table
Immunity related tests
In addition, analyses of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), antinuclear antibody (ANA), anti-double stranded DNA antibody (anti-ds-DNA), ANCA, immunoglobulin, and complement levels were also tested. The ESR and CRP levels were elevated in most of the recruited patients, with median values of 39.5 mm/h and 12.9 mg/dL, respectively. The median value of MPO-ANCA of all patients with MPA was 118.7 RU/mL (range, 28.7–275.7 RU/mL), and PR3-ANCA and MPO-ANCA were both positive in only one patient. ANA was positive in 19 patients (33.34%), and ds-DNA was positive in only 2 patients (3.51%). Many patients had increasing immunoglobulin levels and decreasing C3 levels. MPO-ANCA was positive in all of the MPA patients.
Imaging results and pulmonary function tests
All 57 patients with MPA underwent high-resolution CT scan of the chest. According to their chest CT scan results, 46 (80.71%) patients had interstitial pneumonia and 29 (50.88%) of these patients had pulmonary fibrosis. Twenty-six (45.62%) patients also had complications including emphysema (45.62%), pleural thickening (45.62%), and pleural effusion (19.30%) (Table 1). Twenty-four patients (42.11%) underwent pulmonary function tests; 14 of these 24 patients were found to have restrictive ventilatory dysfunction, 6 patients had obstructive ventilatory dysfunction and small airway dysfunction, and 4 patients had normal results. Echocardiography was also performed on all of our patients; six patients showed pulmonary artery hypertension and two patients had pericardial effusion. Finally, electromyography was performed on only two patients, which revealed peripheral neuropathy in one patient and normal results in the other patient. Most MPA patients had interstitial pneumonia.
Renal biopsy and treatment
Many patients with MPA develop renal injury. Therefore, we also performed renal biopsy in nine patients, and the results showed focal proliferative and necrotizing glomerulonephritis, which was compatible with kidney injury in AAV. After the diagnosis, all patients with MPA were treated with prednisone or methylprednisolone, and cyclophosphamide.
Correlation between IgA and the readmission possibility of MPA patients
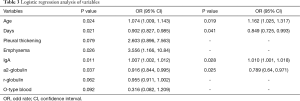
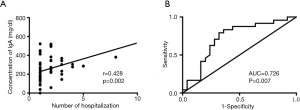
Among the 57 patients, 5 patients were excluded from the readmission analysis because of the short time since their discharge from our hospital. In addition, two patients with MPA who died during their first admission were also excluded. Therefore, we divided the remaining 50 patients with MPA into two groups according to their number of hospitalizations: 22 of these patients had only one hospitalization and were considered as group 1, and the other 28 patients had more than one hospitalization and were considered as group 2. All of the above-mentioned parameters were compared between these two groups. As a result, age (P=0.01), number of days of initial hospitalization (P=0.004), emphysema (P=0.034), IgG level (P=0.003), and a2-globulin level (P=0.036) were all significantly different between the two groups. Then, univariate logistic regression analysis was performed on all the variables shown in Tables 1 and 2. After the analysis, variables with P<0.1 including age (P=0.024), number of days of initial hospitalization (P=0.021), pleural thickening (P=0.079), emphysema (P=0.026), IgA (P=0.011), a2-globulin (P=0.037), r-globulin (P=0.062), and O-type blood (P=0.092) were entered into multivariate logistic regression analysis (forward conditional). The results revealed that age, number of days of initial hospitalization, IgA, and a2-globulin could be entered into the regression analysis. Age had the highest odds ratio (OR) of 1.162 [95% confidence interval (CI): 1.025–1.317, P=0.019], followed by IgA with an OR of 1.010 (95% CI: 1.001–1.018, P=0.028). Hence, age and levels of serum IgA were risk factors for readmission in patients with MPA. The number of days of initial hospitalization and the a2-globulin level were protective factors with ORs of 0.849 (95% CI: 0.725–0.993, P=0.041) and 0.789 (95% CI: 0.64–0.971, P=0.025), respectively (Table 3). Pearson correlation analysis showed that only IgA levels were positively correlated with the number of hospitalizations with a correlation coefficient of 0.428 (P=0.002) (Figure 1A). The ROC curve analysis showed that the AUC was 0.726 (0.578–0.873, P=0.007). Moreover, when the serum levels of IgA were >217.5 mg/dL, the possibility of readmission increased (Figure 1B). These data showed that the level of serum IgA was a risk factor for the readmission of patients with MPA, and correlated with the number of hospitalizations in these patients.
Full table
Discussion
This study had two important findings. First, most of the patients had elevated levels of D-dimers; however, only three patients had venous thrombosis. The coagulation function was normal in most of the patients with MPA in our study group. Second, age and levels of IgA were risk factors for the possibility of readmission in patients with MPA. Furthermore, the number of days of initial hospitalization and the a2-globulin levels were protective factors in these patients. The levels of IgA were positively correlated with the number of hospitalizations.
Venous thrombosis is a new risk factor in patients with MPA (11,16). Allenbach performed a retrospective, systematic analysis of 1,130 patients with systemic vasculitis. Their results showed that venous thromboembolic events (VTEs) occurred in 18 of 236 (7.6%) patients with MPA, and multivariate analysis retained age, male sex, or previous VTE or stroke with motor deficit as factors associated with a higher VTE risk (17). We found that 3 of the 57 patients (5.2%) in this study developed deep vein thrombosis during their first-time admissions; however, we did not perform follow-up for this condition. The D-dimer levels were elevated in most of these patients with MPA, because the endothelial cells were damaged and the coagulation system was activated during disease induction (18,19).
As we have described before, in approximately one-fourth of patients with MPA, active disease persisted or recurred in the first 6 months despite treatment (3). Consequently, many studies were performed to evaluate the risk factors of relapse or readmission. A previous study revealed that an increase in ANCA level during remission was associated with a risk of disease relapse. An increase in the ANCA level may be useful for guiding treatment decisions in appropriate subsets of patients with AAV (20). In addition, many novel risk factors were also reported, including exposure to farms and farm animals (21). Within 5 years of diagnosis of Wegener’s granulomatosis or MPA, 14% of patients will have a 5-year cardiovascular risk event (22). As for the prognosis of patients with MPA, evidence showing the short-term prognosis of MPA is weak. The mortality of MPA is mainly concentrated in the first months after diagnosis. The long-term prognosis of MPA is less severe; however, relapses are frequent. Early diagnosis, early treatment according to risk factors, and a longer follow-up of patients are needed (23). Additionally, age ≥65 years and development of pulmonary infections after immunosuppressive treatments were identified as risk factors for death during hospitalization (24), especially infection. A previous study analyzed the infection conditions of 61 patients with MPA. A total of 61 patients developed 147 infections, showing that infectious events are frequent. The results also showed that relapse and infection shared similarities during the course of vasculitis (25). Hence, in our study, we did not analyze the reasons for readmission of patients with MPA. The IgG levels were identified as factors associated with infectious complications (14), and no research has been reported about the correlation between IgA and patients with MPA. Previous study reported a pedipediatric case of microscopic polyangiitis with skin manifestations resembling vesiculobullous type erythema elevatum diutinum with immunoglobulin A antineutrophil cytoplasmic antibody. So far, no direct evidence proved the relation between Ig A levels and MPA patient. More investigations still need to be performed.
This study has several limitations. The study population was relatively small, and more patients should be recruited. Many test results were missing, and only nine patients underwent renal biopsy. However, we still had a novel finding. This is the first report to demonstrate that the IgA levels of patients with MPA on their first-time admissions were correlated with the possibility of readmission in these patients.
Conclusions
In conclusion, most patients with MPA have high levels of D-dimers. Age and serum IgA are risk factors for the readmission of patients with MPA.
Acknowledgements
Funding: This work was supported by the National Science Foundation of China (81500003) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ID: ZYLX201312).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethics committee of our hospital approved the study protocol.
References
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1-11. [Crossref] [PubMed]
- Huang H, Wang YX, Jiang CG, et al. A retrospective study of microscopic polyangiitis patients presenting with pulmonary fibrosis in China. BMC Pulm Med 2014;14:8. [Crossref] [PubMed]
- Miloslavsky EM, Specks U, Merkel PA, et al. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2013;65:2441-9. [Crossref] [PubMed]
- Daikeler T, Kistler AD, Martin PY, et al. The role of rituximab in the treatment of ANCA-associated vasculitides (AAV). Swiss Med Wkly 2015;145:w14103. [PubMed]
- Alberici F, Smith RM, Jones RB, et al. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology (Oxford) 2015;54:1153-60. [Crossref] [PubMed]
- Timlin H, Lee SM, Manno RL, et al. Rituximab for remission induction in elderly patients with ANCA-associated vasculitis. Semin Arthritis Rheum 2015;45:67-9. [Crossref] [PubMed]
- Crickx E, Machelart I, Lazaro E, et al. Intravenous Immunoglobulin as an Immunomodulating Agent in Antineutrophil Cytoplasmic Antibody-Associated Vasculitides: A French Nationwide Study of Ninety-Two Patients. Arthritis Rheumatol 2016;68:702-12. [Crossref] [PubMed]
- Miloslavsky EM, Specks U, Merkel PA, et al. Outcomes of nonsevere relapses in antineutrophil cytoplasmic antibody-associated vasculitis treated with glucocorticoids. Arthritis Rheumatol 2015;67:1629-36. [Crossref] [PubMed]
- Jones RB, Furuta S, Tervaert JW, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis 2015;74:1178-82. [Crossref] [PubMed]
- Ward ND, Cosner DE, Lamb CA, et al. Top Differential Diagnosis Should Be Microscopic Polyangiitis in ANCA-Positive Patient with Diffuse Pulmonary Hemorrhage and Hemosiderosis. Case Rep Pathol 2014;2014:286030. [Crossref] [PubMed]
- Kechaou I, Cherif E, Boukhris I, et al. Microscopic polyangiitis: A little-known new risk factor of venous thrombosis. J Mal Vasc 2015;40:406-7. [Crossref] [PubMed]
- Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488-94. [Crossref] [PubMed]
- Wada T, Hara A, Arimura Y, et al. Risk factors associated with relapse in Japanese patients with microscopic polyangiitis. J Rheumatol 2012;39:545-51. [Crossref] [PubMed]
- Kitagawa K, Furuichi K, Sagara A, et al. Risk factors associated with relapse or infectious complications in Japanese patients with microscopic polyangiitis. Clin Exp Nephrol 2016;20:703-711. [Crossref] [PubMed]
- Kawai H, Banno S, Kikuchi S, et al. Retrospective analysis of factors predicting end-stage renal failure or death in patients with microscopic polyangiitis with mainly renal involvement. Clin Exp Nephrol 2014;18:795-802. [Crossref] [PubMed]
- Stassen PM, Derks RP, Kallenberg CG, et al. Venous thromboembolism in ANCA-associated vasculitis--incidence and risk factors. Rheumatology (Oxford) 2008;47:530-4. [Crossref] [PubMed]
- Allenbach Y, Seror R, Pagnoux C, et al. High frequency of venous thromboembolic events in Churg-Strauss syndrome, Wegener's granulomatosis and microscopic polyangiitis but not polyarteritis nodosa: a systematic retrospective study on 1130 patients. Ann Rheum Dis 2009;68:564-7. [Crossref] [PubMed]
- Hergesell O, Andrassy K, Nawroth P. Elevated levels of markers of endothelial cell damage and markers of activated coagulation in patients with systemic necrotizing vasculitis. Thromb Haemost 1996;75:892-8. [PubMed]
- Ohdama S, Aoki N. Increase of plasma thrombomodulin in systemic vasculitis. Thromb Haemost 1997;77:609. [PubMed]
- Yamaguchi M, Ando M, Kato S, et al. Increase of Antimyeloperoxidase Antineutrophil Cytoplasmic Antibody (ANCA) in Patients with Renal ANCA-associated Vasculitis: Association with Risk to Relapse. J Rheumatol 2015;42:1853-60. [Crossref] [PubMed]
- Willeke P, Schlüter B, Sauerland C, et al. Farm Exposure as a Differential Risk Factor in ANCA-Associated Vasculitis. PLoS One 2015;10:e0137196. [Crossref] [PubMed]
- Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener's granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011;63:588-96. [Crossref] [PubMed]
- Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, et al. Overall survival, renal survival and relapse in patients with microscopic polyangiitis: a systematic review of current evidence. Rheumatology (Oxford) 2011;50:1414-23. [Crossref] [PubMed]
- Tanaka M, Koike R, Sakai R, et al. Pulmonary infections following immunosuppressive treatments during hospitalization worsen the short-term vital prognosis for patients with connective tissue disease-associated interstitial pneumonia. Mod Rheumatol 2015;25:609-14. [Crossref] [PubMed]
- Debouverie O, Roy-Péaud F, Béraud G, et al. Infectious events during the course of systemic necrotizing vasculitis: a retrospective study of 82 cases. Rev Med Interne 2014;35:636-42. [Crossref] [PubMed]


