Clinicopathological factors influenced the prognosis of surgically resected pulmonary pleomorphic carcinoma
Introduction
Pulmonary pleomorphic carcinoma (PC) is a rare pulmonary histopathological type of primary lung cancer occupying about 0.1–0.4% (1). In 1999, WHO defined PC is a poorly differentiated non-small cell lung carcinoma (NSCLC) namely a squamous cell carcinoma, adenocarcinoma, or undifferentiated non-small cell carcinoma that contains at least 10% spindle and/or giant cells or a carcinoma consisting only of spindle and giant cells (2). According to the WHO report, PC makes an unfavorable prognosis because of its properties of resisting radiation and chemotherapy, and its aggressive growth (3-6). Since the studies of patients with PC was small and rare, the factors affecting their survival after having the pulmonary resection for PC and its clinicopathological characteristics are still undiscovered.
This study aims at improving the understanding of the PC and exploring an adequate therapeutic strategy. Here we examined the correlation between clinicopathological factors and prognosis about 24 pulmonary PC patients who had surgical resection at our hospital and reviewed the previous reports of pulmonary PC.
Methods
We reviewed the past medical records of our institute, Nagoya City University Hospital. We operated 926 cases of NSCLC from January 2004 to December 2013. Out of those 926 patients, 24 (2.6%) were diagnosed as pulmonary PC. We closely examined the 24 cases in order to find the clinicopathological features of PC. The study was approved by the Institutional Review Board of Nagoya City University Hospital (No. 48), and a written consent was obtained each patient to use their clinicopathological data for research.
Since a diagnosis of PC before the surgery was quite difficult, most cases were diagnosed after a surgical resection. In our cases, one case was diagnosed as PC, 4 cases as adenocarcinoma, 1 case as squamous cell carcinoma, 7 cases as NSCLC, and the rest 11 cases were not diagnosed at all before having a surgery.
The analysis of survival curves was based on the Kaplan-Meier method and univariate log-rank test. Overall survival (OS) was calculated from the date of having a surgery to that announcing a death. Progression free survival (PFS) was calculated from the date of having a surgery to that of identifying a progression disease or death for any cause. Significance was defined as a probability value of less than 0.05. All of the data were analyzed with the EZR software (7).
Results
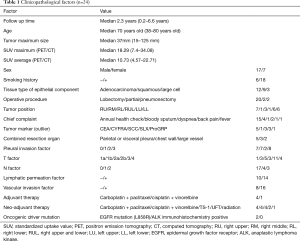
The patient’s characteristics are shown in Table 1. Briefly, the age range of the patients consisting of 17 males and 7 females was from 38 to 80 (median 70) years old. Eighteen patients were current- or ex-smokers, the size of tumor was from 15 to 125 (median 37) mm, and the pathological stage was 3 at IA, 1 at IB, 4 at IIA, 10 at IIB, 3 at IIIA and 2 at IV. Two patients had pneumonectomy, 20 lobectomy, and 2 partial resection. As the combined resected organ, 3 patients were chest wall, 5 were parietal resection and 2 were large vessel. The immunohistochemical examination showed that 7 patients were pl0, 7 were pl1, 2 were pl2, and 8 were pl3. Fourteen patients were lymphatic permeation positive, and 10 were negative. Sixteen patients were vascular invasion positive, and 8 were negative. The tissue type of epithelial components was adenocarcinoma in 12 (50%) patients, squamous cell carcinoma in 9 (37.5%), and large cell carcinoma in 3 (12.5%). The nodal status was classified as pN0 disease in 17 (71%) patients, pN1 in 4 (17%), and pN2 in 3 (13%). We investigated the epidermal growth factor receptor (EGFR) mutation by a direct sequence, and anaplastic lymphoma kinase (ALK) translocation by immunohistochemistry. We identified two cases as EGFR L858R mutation, but no case as ALK translocation. The median of Standardized Uptake Value (SUV) max and SUV average by 2-[18R]-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography (PET) was 18.29 and 10.73 for each in these 22 PC patients. 18F-FDG uptake of PC patients was higher than those of usual histological types of lung cancer (8).
Full table
The 5-year OS was 54.7% (Figure 1A). Twenty-two patients underwent a complete resection, but two had an incomplete resection because of remaining at the parietal pleural by pathological examination in one patient and malignant pleural effusion by cytology in the other. Nine patients were recurrent. The median period until recurrence was 132 days (27 to 725 days), and recurrence within 6 months after having a resection occurred in 7 patients. The median survival time after confirming an initial relapse was 186 days (from 21 to 603 days). Eight cases were distant metastasis (2 brain, 2 bone, lung, liver, adrenal, and small intestine) and 1 case was pleural dissemination.
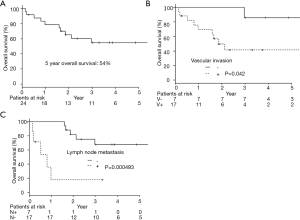
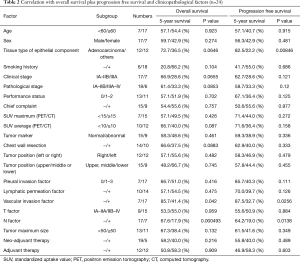
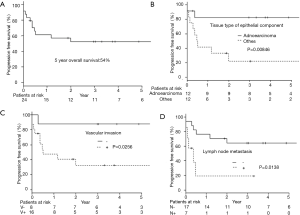
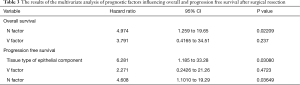
As a result of our analysis, we found that the correlation between OS and clinicopathological factors; vascular invasion and lymph node metastasis had a harmful effect on the OS (Table 2, Figure 1B,C). The 5-year PFS was 52.4% (Figure 2A). The analysis of the correlation between PFS and clinicopathological factors revealed that the tissue type of epithelial component, vascular invasion, and lymph node metastasis affected the PFS (Table 2, Figure 2B,C,D). On the whole, the lymph node metastasis was the factor of affecting the OS, and vascular invasion and lymph node metastasis were the factor of affecting the PFS (Table 3).
Full table
Full table
Discussion
Pulmonary PC is a rare histological type of lung carcinoma. Hence there is few large-scale clinical data in comparison to other common histological types of lung carcinoma. In addition, the prognostic factors and therapeutic strategy for pulmonary PC have not been found yet. The prognosis and the predictors for the long-term survival of pulmonary PC were in fact controversial. Previously pulmonary PC is considered as taking an aggressive clinical course than other NSCLCs (3-6). However, Yamamoto et al. (9) and Nakajima et al. (10) reported that the prognosis was not worse than other types of histology. In this study, we examined the correlation between the prognosis of pulmonary PC and clinicopathological factors. The 5-year OS of the 24 patients including several pathological stages was 54.7%, and the 5-year PFS was 52.4%. In comparison with other tissue types of lung cancer, the prognosis was not so poor even taking into consideration the survival curve including several progression stage (11). Nine patients faced the relapse which happened within 2-years after having a surgery. In the cases of pulmonary PC, there are two groups; one has high grade malignant property with aggressive invasion, and the other has a good prognosis if a complete resection is held.
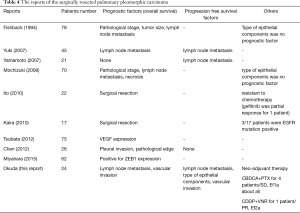
We investigated the relation between 21 clinicopathological factors and the prognosis (OS and PFS). Since the pulmonary PC is heterogeneous, it is difficult to diagnose PC before a surgical resection. In order to predict PC before a surgery, the PET-CT in the investigated clinicopathological factors might have one possibility. The SUV max and SUV average are clearly higher than other histological types of NSCLCs (12). By taking into account the prognosis, a complete resection was the most important factor. The PC was located mainly at the peripheral lung field, invading to the parietal pleura and chest wall. However, the chest wall invasion was not a prognostic factor. In fact, the recurrence at the surgically resected edge of a chest wall was not recognized in this study. On the other hand, the lymph node metastasis and the vascular invasion were the factors deeply affected OS, and the lymph node metastasis was the only significant prognostic factor by multivariate analysis. The lymph node metastasis, the type of epithelial components, and vascular invasion affected PFS, and the lymph node metastasis and vessel invasion were significant factors by multivariate analysis. We also reviewed the recent reports of PC at Table 4 (3,5,6,8,13-17). Just like some of the reports revealed that the lymph node metastasis was associated with a poor prognosis, our result followed those preceding reports. In this study, we didn’t investigate about the sarcomatoid component as clinicopathological factor. Yuki et al. (5) reported the subtype of sarcomatous elements did not influence prognosis.
Full table
According to the investigation of the recurrent cases, the nine cases showed a relapse within 2years after a surgery, and most recurrent forms were distant metastases. Hence we need to find the effective chemotherapeutic drug and regimen as a neo-adjuvant and adjuvant therapy. In the one case of this study, cisplatin and vinorelbine were effective as a neo-adjuvant therapy (2 courses before surgery), and the pathological examination discovered Ef. 2b. Since the vascular invasion was associated with a poor prognosis, there is a possibility that vascular endothelial growth factor (VEGF) inhibitor would be effective for PC patients. Regarding the genetic profile of PC, some cases of EGFR activating mutation have been reported (13). Kobayashi et al. reported the case of transformation to sarcomatoid carcinoma in ALK-rearranged adenocarcinoma (18). Here the known genetic abnormalities associated with histological components, especially tumors with adenocarcinoma components, recommends molecular testing. We also investigated EGFR mutation and ALK translocation of the 14 cases and 24 cases each in this study. We found EGFR L858R mutation in 2 cases, and the 2 cases were treated by EGFR-TKI when we confirmed the recurrence. Erlotinib was not effective against both of these 2 patients. Including VEGF expression and the therapeutic efficacy, we should research the biological futures of PC, and develop an effective molecular targeted therapy. The research of molecular pathways will uncover the oncogenic driver mutation of pulmonary PC, and moreover give rise to an effective therapy for pulmonary PC.
In the study, we examined the relations between 21 clinicopathological factors and the prognosis in order to clarify the characteristics of pulmonary PC. The prognosis of pulmonary PC patients with lymph node metastasis, vascular invasion, and the tissue type of epithelial component worsened after their surgical resection. We have to find an effective chemotherapeutic drug or molecular targeted drug, and we should treat pulmonary PC patients by an adequate adjuvant therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chang YL, Lee YC, Shih JY, et al. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer 2001;34:91-7. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth edition. WHO Classification of Tumours 2015;7.
- Fishback NF, Travis WD, Moran CA, et al. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 1994;73:2936-45. [Crossref] [PubMed]
- Raveglia F, Mezzetti M, Panigalli T, et al. Personal experience in surgical management of pulmonary pleomorphic carcinoma. Ann Thorac Surg 2004;78:1742-7. [Crossref] [PubMed]
- Yuki T, Sakuma T, Ohbayashi C, et al. Pleomorphic carcinoma of the lung: a surgical outcome. J Thorac Cardiovasc Surg 2007;134:399-404. [Crossref] [PubMed]
- Mochizuki T, Ishii G, Nagai K, et al. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008;32:1727-35. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Han B, Lin S, Yu LJ, et al. Correlation of 18F-FDG PET activity with expressions of survivin, Ki67, and CD34 in non-small-cell lung cancer. Nucl Med Commun 2009;30:831-7. [Crossref] [PubMed]
- Yamamoto S, Hamatake D, Ueno T, et al. Clinicopathological investigation of pulmonary pleomorphic carcinoma. Eur J Cardiothorac Surg 2007;32:873-6. [Crossref] [PubMed]
- Nakajima M, Kasai T, Hashimoto H, et al. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer 1999;86:608-16. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Evolution of surgical outcomes for nonsmall cell lung cancer: time trends in 1465 consecutive patients undergoing complete resection. Ann Thorac Surg 2004;77:1926-30; discussion 1931.
- Kaira K, Endo M, Abe M, et al. Biologic correlates of 18F-FDG uptake on PET in pulmonary pleomorphic carcinoma. Lung Cancer 2011;71:144-50. [Crossref] [PubMed]
- Kaira K, Horie Y, Ayabe E, et al. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol 2010;5:460-5. [Crossref] [PubMed]
- Ito K, Oizumi S, Fukumoto S, et al. Clinical characteristics of pleomorphic carcinoma of the lung. Lung Cancer 2010;68:204-10. [Crossref] [PubMed]
- Tsubata Y, Sutani A, Okimoto T, et al. Tumor angiogenesis in 75 cases of pleomorphic carcinoma of the lung. Anticancer Res 2012;32:3331-7. [PubMed]
- Chen F, Sonobe M, Sato T, et al. Clinicopathological characteristics of surgically resected pulmonary pleomorphic carcinoma. Eur J Cardiothorac Surg 2012;41:1037-42. [Crossref] [PubMed]
- Miyahara S, Hamasaki M, Hamatake D, et al. Clinicopathological analysis of pleomorphic carcinoma of the lung: diffuse ZEB1 expression predicts poor survival. Lung Cancer 2015;87:39-44. [Crossref] [PubMed]
- Kobayashi Y, Sakao Y, Ito S, et al. Transformation to sarcomatoid carcinoma in ALK-rearranged adenocarcinoma, which developed acquired resistance to crizotinib and received subsequent chemotherapies. J Thorac Oncol 2013;8:e75-8. [Crossref] [PubMed]


