An update on intraoperative three-dimensional transesophageal echocardiography
Clinical cardiac ultrasound emerged after World War II, discovered with the help of naval sonar technology. Initially, images consisted of a single ultrasound vector of ultrasound displayed over time—“M-Mode” imaging (1). Over the next several years, technological advances over the next several years allowed for the introduction of 2D (1970s) and 3D (1990s) imaging into clinical practice. Transesophageal echocardiography (TEE) developed in 1970s overcame the limitations of transthoracic echocardiographic (TTE) images (2). Notably, in 1980, TEE became standard practice for the first time in cardiac operating rooms to facilitate surgical decision-making (1). In the 1990s, the first 3D images of the mitral valve (MV) were created using the rotational scan plane technology during post processing, However, this was limited by difficult acquisition methods and cumbersome analysis software (2). The advent of matrix transducers made the clinical use of 3D TEE possible. Real-time three-dimensional (RT-3D) TEE became widely used intraoperatively and second and third generation advancements in TEE transducers allowed for increased spatial and temporal resolution. With faster processing and quantification. RT-3D TEE has become a simple and quick imaging modality, increasingly practiced by experienced echocardiographers.
Technical aspects of 3D imaging
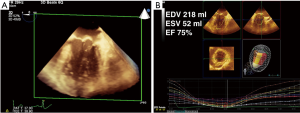
RT-3D TEE has its limitations and it is a tool that must be learned. Because of the increased amount of data points required to generate a 3D image or volume, the major limitation of 3D imaging is poor temporal resolution. Resolution may be improved by narrowing the sector of acquisition because the width of the sector is inversely proportional to the temporal resolution (3). Smaller structures, such as the aortic, mitral, tricuspid and pulmonic valves can be imaged in real time using a single heart beat with adequate temporal resolution if the sector is narrow enough to contain the valve of interest. Larger structures such as the left ventricle (Figure 1) must be imaged with a wide-angle sector, and the temporal resolution is improved by using gated acquisitions (dividing the imaging sector into 2, 4, or 6 slices and “stitching” the images back together) (1,3). Multi-beat acquisitions can also be used to increase the temporal and spatial resolution, especially when using color flow Doppler (CFD).
Gated acquisitions are more difficult to perform, and there are several challenges intraoperatively. Firstly, they require a motionless patient, apnea, absence of electrocautery, and a regular heart rate for the duration of the multi-beat acquisition (usually 2, 4, or 6 beats) (1). Any movement causes “stitching” to occur, an artifact whereby 3D volume slices are not lined up, resulting in a distorted image. Depending on the area of interest, different RT-3D TEE modes can be used to maximize the spatial and temporal resolution of the area of interest. Clinically, 3D imaging has been used intraoperatively in MV surgeries, percutaneous interventions [transcatheter aortic valve interventions (TAVIs), left atrial appendage (LAA) occlusion devices, and edge-to-edge MV repair devices (Mitraclip®)], as well as placement of atrial septal defect (ASD) and paravalvular leak (PVL) closure devices (3).
Applications of 3D TEE
Left ventricular volume and function
Left ventricular (LV) assessment and quantification is one of the most important perioperative applications of clinical TEE. Routine 2D echocardiography is widely accepted and used to evaluate LV size and function. The most commonly used method and recommended technique for 2D echocardiographic volume calculations is the biplane method of disks summation (MOD), i.e., the modified Simpson’s rule (4).
This technique is commonly applied in the mid-esophageal four chamber (ME 4Ch) and ME 2Ch views of the LV to determine the end diastolic volume (EDV), end systolic volume (ESV), stroke volume (SV), and ejection fraction (EF). 2D images of the LV can be foreshortened in these views, thus making the measurements less accurate. 3D TEE quantification overcomes the limitations of foreshortening by calculating LV absolute volumes without any geometric assumptions. Additionally, 3D is also useful in cases where the shape of the LV is abnormal, such as in post myocardial infarction (MI) LV aneurysms, when the assumptions LV of geometry of the biplane MOD do not hold true.
3D LV volume and EF determination have been studied against 2D methods outside the operating room, and found to be accurate when compared to magnetic resonance imaging (MRI). There is good correlation between MRI and 3D TTE. A recent meta-analysis pooled twenty-three studies which included 1,638 echocardiograms, and found that the standard deviations (SD) for 3D TTE were −19.1±34.2 mL for EDV, −10.1±29.7 mL for ESV, and −0.6±11.8% for EF compared to MRI, and that 3D TTE measurements correlated better to MRI than 2D measurements (P=0.01 for both EDV and ESV) (5). In the operating room, 3D and 2D TEE parameters of EF, ESV, and EDV were compared and 3D values were found to be no different in terms of EF, but 3D TEE had larger values for EDV and ESV. Respectively, the larger values of EDV and ESV were not significant enough to change the LV size classification (normal, mildly dilated, moderately dilated, or severely dilated) in 98.8% of cases for indexed EDV and 92.8% of cases for indexed ESV. Intraoperative 3D LV quantification was associated similar acquisition times (P=0.805; pairwise difference =2 seconds [95% PIs, −20 to 35 seconds]), but longer analysis times (<0.001; pairwise difference =117 seconds [95% PIs, 66 to 197 seconds]), but remained feasible. (6) In light of this, the new perioperative American Society of Echocardiography (ASE) recommendations in 2015 states that in patients with good image quality, 3D EF measurements are accurate and reproducible, and should be performed (3) (Figure 1).
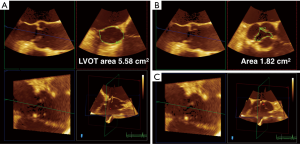
3D TEE may also improve the accuracy of quantitative Doppler methods in the operating room. Quantitative assessment of LV stroke volume (SV) is performed by multiplying the spectral Doppler trace of left ventricular outflow tract (LVOT) velocity, the velocity time integral (VTI), by the LVOT area. The LVOT area is calculated in 2D TEE by using the LVOT diameter taken in the ME long axis (ME LAX) view, and using the area equation for a circle to determine an area. However, multiple studies have shown that the LVOT is usually elliptical, with the short axis of the ellipse corresponding to the LVOT diameter obtained in the ME LAX view. Gasper et al. found that LVOTs were oval in 96% of the 50 patients studied, with a mean eccentricity index (diameter 2/diameter 1) of 1.26±0.09 by cardiac computed tomographic angiography (CCTA). Compared with CCTA, 2D TTE systematically underestimated LVOT area by 17%±16%. Therefore, there is an inherent error built into the stroke volume equation, with the 2D LVOT area usually underestimating the real LVOT area. However, because of poor 3D TTE image quality, the study only found moderate correlation between CCTA and 3D TTE LVOT area because of poor 3D TTE image quality (7). TEE has better imaging windows which to generate higher quality 2D and therefore 3D images and may overcome this problem (7). In high quality 3D TEE acquisitions, the LVOT area can be approximated by lining up two orthogonal long-axis planes and tracing the cross-sectional plane in short axis using quantification software (Figure 2). Using 3D LVOT area to calculate SV should therefore improve the accuracy of cardiac output measurements intraoperatively (SV × heart rate = CO).
Mitral valve
Mitral valve repair
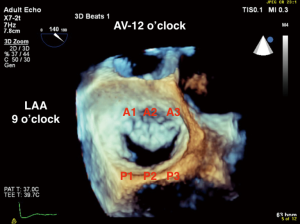
RT-3D TEE has changed the way the MV is evaluated intraoperatively. Due to its posterior anatomic position in the heart (close to the esophagus) the MV can be easily evaluated in 2D and 3D without shadowing or artifact. A narrow imaging sector is usually sufficient to capture the entire valve, and a single beat acquisition often provides adequate spatial and temporal resolution to evaluate MV anatomy and pathology. The intraoperative echocardiographer can effectively with the surgeon by rotating the 3D image of the MV to the surgeon’s en face view in real time, with the aortic valve (AV) in the 12 o’clock position, LAA in the 9 o’clock position, and the coronary sinus in the 3 o’clock position (8,9) (Figure 3). Similarly, the post-operative evaluation of the valve using 3D TEE with color Doppler may aid in determination of number and location of paravalvular leaks (PVLs) if present (10).
Multiple studies have shown that 3D imaging of the MV is superior to 2D in the qualitative assessment of degenerative mitral regurgitation (MR) (11-13) (Figure 4). Grewal et al. found that expert echocardiographers were able to diagnosis P1, A2, A3, and bileaflet disease with 3D imaging better than 2D imaging (11). Biaggi et al. found that 3D TEE assessment of MV anatomy in degenerative MR accurately reflected surgical anatomy, and predicted the complexity of MV repair. Specifically, when 2D and 3D TEE measurements (prolapsed segments, leaflet heights, and annular dimensions) were compared to surgical measurements, 3D TEE was more accurate (92–100%) than 2D TEE (80–96%) in identifying prolapsed segments. In addition, 2D TEE significantly overestimated the height of the posterior segment P1 and the anterior segment A2, while 3D TEE measurements correlated to surgical measurements (14). Finally, 3D quantitative measurements of the number of prolapsed segments (one vs. two to four vs. five or more) and the enlargement of the annular anteroposterior and commissural diameters predicted more complex MV repair using larger annuloplasty bands and a greater number of neochords (14). Therefore, 3D imaging of the MV is superior in demonstrating valvular anatomy, and particularly identifying specific MV pathology in complex disease, and aids in planning surgical repairs.
Mitral valve area
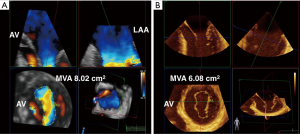
3D TEE has changed the determination of MV area by planimetry has also changed with the advent of 3D TEE. In 2D planimetry, the mitral valve area (MVA) is measured by tracing the MV orifice outline during mid-diastole in the basal trans-gastric short axis (TG SAX) view. This method of measurement is anatomic, does not depend on flow or chamber compliance compared to other methods of measuring MVA i.e., using mean gradient or pressure half-time. 2D limitations include the difficulty in finding the smallest orifice of the MV and ensuring that the measured orifice is orthogonal to the direction of flow. These limitations may be addressed by 3D imaging using 3 simultaneous orthogonal places in multiplanar reconstruction (MPR) which improves the accuracy of measurement of the narrowest orifice (albeit at a lower temporal resolution). It is important to note that highly gained 3D acquisitions can underestimate the orifice area due to the MV appearing falsely smaller than it actually is (12). Using color flow Doppler (CFD) helps to locate the functional orifice, which in stenotic valves, occurs more distally than the anatomic orifice. The functional orifice obtained by 3D CFD should correlate with other methods of MVA calculations based on maximal velocity such as pressure half time, proximal isovelocity surface area (PISA) method, and continuity equation. The use of 3D TEE may be useful in mixed MR/Mitral stenosis (MS) disease as a less flow dependent measurement of MVA (15) (Figure 5).
Percutaneous mitral valve edge-to-edge repair
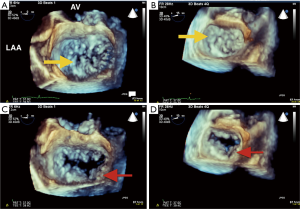
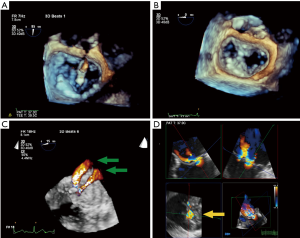
RT-3D TEE has proven to be an essential tool for procedural imaging. As in MV surgery, 3D TEE is useful to appreciate the detailed anatomy of the MV en face, assess the etiology and origin of MR, and guide edge-to-edge percutaneous MV repair. 3D TEE may be superior in detecting tissue defects such as clefts, which affect the feasibility of the percutaneous repair procedure, and which are more difficult to recognize using 2D TEE. The en face view may also provide critical information on clinical pitfalls. They include: shorter or tethered leaflet segments, and larger flail gap segments, and greater coaptation depths, that would preclude a good leaflet grasp during percutaneous MV repair. RT-3D TEE is particularly useful in aligning procedural catheters and clip delivery system in relation to the MV once the guide catheter is advanced across the interatrial septum. It can help orient the medial, lateral, anterior, and posterior adjustments of the clip delivery system. This ensures that the clip and the delivery system is perpendicular to the plane of coaptation of the MV. RT-3D TEE can be used to orient the clip (and its arms) before deployment, ideally aligned perpendicularly to the coaptation line in the open position (Figure 6A). After clip deployment, 3D TEE views are used to assess the result of the repair by visualizing the MV orifices either from the atrial or ventricular perspective (Figure 6B). The addition of CFD can aid in determining the amount of residual MR and the region from which it originates. If additional clips are required due to residual MR, 3D CFD imaging can locate the area of the largest jet, which should be the target of the next clip (16) (Figure 6D). If multiple clips are used, 3D TEE to visualize clips in relation to one another orientation of one clip with another, which are hopefully positioned in parallel. Currently, post Mitraclip® repair, MVA is measured by 2D planimetry at the level of the clip in the basal TG SAX view and assessed by the mean gradient through the valve (16). A potential 3D application would be to measure the functional orifices of the valve using 3D MPR with CFD; however, this method has not been validated for MVA post edge-to-edge repair. Following clip(s) deployment, especially in the-delete cases with residual MR, 3D functional assessment of valve area using multi-beat acquisition quantification software with CFD can help evaluate for mitral stenosis and the feasibility of placing another clip.
Mitral valve paravalvular Leak (PVL) closure
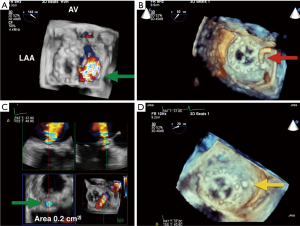
Up to 5% of patients post MV replacement have significant PVLs that require clinical intervention due to heart failure symptoms or hemolytic anemia (17). For patients at prohibitively high risk for re-operation, catheter-based closure of paravalvular MR is an attractive alternative. RT-3D TEE has been used to help define, locate, grade the severity, and guide PVL closure procedures. RT-3D has emerged as the preferred imaging modality over 2D TEE for evaluation of morphology of PVLs due to its ability to better define their size and shape (18). Gated acquisition of 3D with CFD data sets can partially overcome the low temporal resolution of this imaging modality and help determine the regurgitant orifice shape, the number of jets, and the effective regurgitation orifice (ERO) of the PVL at the level of the sewing ring. 3D TEE can identify the exact location of the orifice of paravalvular regurgitation around the MV and guide the proceduralist in accessing the PVLs based on its location (18) (Figure 7). The location of the PVL can be described both by the clock-face method as described earlier (AV is 12 o’clock and the left atrial appendage is 9 o’clock), or anatomic language in quadrants (medial, lateral, anterior medial, anterior lateral, posterolateral, posteromedial etc.) (8,17). For grading PVL severity, Franco et al. evaluated the usefulness of planimetry of the ERO of the PVL by 3D CFD TEE compared with 3D planimetry without CFD to quantify the severity of the PVL and assess PVL closure success (Figure 7C). The authors found that 3D CFD ERO measures correlated better with the degree of paravalvular regurgitation than 3D ERO without CFD. An ERO major diameter ≥0.65 cm showed a positive predictive value of 87.1% and a negative predictive value of 94% in diagnosing degree III and IV paravalvular regurgitation. Additionally, the authors found that closure device undersizing according that closure device undersizing according to 3D color ERO length was significantly associated with PVL closure failure (P=0.007). These findings suggest that under sized dimensions of the PVL calculated with 3D ERO may predict the technical failure of percutaneous closure procedures (18).
Aortic valve
Aortic valve area
3D TEE has been applied to the determination of aortic valve area (AVA) in aortic stenosis by using 3D planimetry and by applying 3D planimetry of LVOT area to the continuity equation ([LVOT area multiplied by the spectral trace of LVOT VTI divided by the spectral trace of continuous wave Doppler (CWD) through the AV]. Multiple studies have shown the superiority of 3D TEE in determining AVA in aortic stenosis (AS) by 3D planimetry over 2D planimetry (8) (Figure 2B). Saitoh et al. showed that 3D TEE planimetry of AVA correlated better with continuity equation derived AVA than 2D planimetry (19). The advantage of 3D is clear for planimetered valve area: in MPR, the orthogonal plane to the two long axis planes can be used to find the smallest valve area (4). This method, though not used the guidelines as diagnostic criteria for AS, may be used as corroborating evidence in situations where the patient is the on the border of moderate to severe AS.
The continuity-derived AVA is used as diagnostic criteria for the grading of AS. Calculation of the AVA by using a 3D planimetry of LVOT area has been demonstrated to be more accurate than using 2D LVOT area (generated from the LVOT diameter) (Figure 2A). This is not a surprise given the studies mentioned earlier showing that the 2D LVOT area and therefore AVA underestimated CCTA measurements by 17% (7). Jainandunsing et al. compared AVA derived from the continuity equation with LVOT areas obtained from intraoperative 2D and 3D measurements, and categorized severity of AS as mild, moderate, or severe. The authors found that 2D methods underestimated LVOT area by 21% (P<0.05) compared with 3D planimeter of LVOT area (4.1±0.1 cm2). Out of 66 patients, 8 patients (12%) who had originally been classified as severe AS by the 2D method were reclassified as moderate AS by the 3D method (P<0.001) (20). Therefore, 3D-derived measurements of AVA are not only more accurate, but have more the potential to impact surgical decision-making in AS.
Transcatheter aortic valve interventions (TAVIs)
3D TEE is an important imaging modality for AV annular sizing for TAVRs. Cross-sectional area (CSA) measurement of the aortic annulus is required in the evaluation of patients for TAVR who have severe AS. Improvements in post-processing software have allowed 3D TEE annular sizing to be a surrogate for CT and cardiac MRI. This is particularly useful in patients with renal insufficiency or failure, where CT/MRI are relatively contraindicated due to the iodinated contrast load that could irreversibly damage their kidneys (21).
3D TEE measurements of the aortic annulus have been used as an alternative to multi-detector row computed tomography (MDCT) (Figure 8). Recent studies (2013–2016) comparing aortic annular cross-sectional area measurements using 3D TEE direct planimetry of the reconstructed cross-sectional view of the annulus and have been shown to correlate, but underestimate MDCT-measured annular areas by 9–20% (21). The echocardiographer can use the same techniques for 3D planimetry of the MVA, AVA, and LVOT areas, by lining up the annular plane in 2 orthogonal long axis views and then tracing the 3D area in the SAX view.
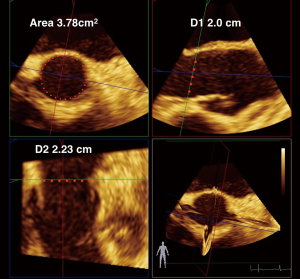
The evolution of 3D software has allowed for a truer measurement of the aortic annular area. Khalique et al. describes a novel technique of 3D TEE annular sizing that corresponds better to MDCT, underestimating the AV annulus by only 1.9% compared to the previous studies. The authors applied the Philllips MVQ (mitral valve quantification semi-automated software) in an off-label use to quantify the aortic annulus, compared the values to direct 3D planimetry of aortic annular area measurements in SAX image, and compared values from both measurements to the gold standard of MDCT. Both direct and semi-automated methods underestimated annular area by MDCT (P<0.05). All methods highly correlated (R=0.88–0.93, P<0.0001), but the 3D TEE semi-automated method underestimated less the annular area (1.9%) versus 3D TEE direct planimetry (3.2%), compared to MDCT (21). These results are both better than underestimations cited in prior publications and validates 3D TEE as a viable alternative to MDCT in sizing the aortic annulus.
Tricuspid valve
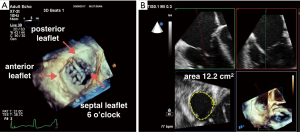
3D TEE is valuable in evaluation and identification of tricuspid valve (TV) leaflets and anatomy by eliminating the need for mental reconstruction of 2D images to understand the anatomy and pathology of the valves. There have not been studies quantifying the advantages of using 3D for TV assessment. Despite this, the 2013 ASE guidelines of a comprehensive TEE exam includes recommends obtaining a 3D single beat, narrow sector acquisition to create an en face view of the TV that has the septal leaflet facing the 6 o’clock position (3) (Figure 9A). It further states that 3D en face views may be particularly helpful in determining the etiology of TV regurgitation such as leaflet prolapse, perforation, or vegetation. 3D TEE may also be used to localize the origin of regurgitation jets, and perform 3D planimetry of the TV to assess severity of TV stenosis (3). Intuitively, 3D quantification of the TV may also improve the accuracy of 2D quantitative Doppler methods of the right ventricle (RV), similar to 3D methods in relation to the LV and LV stroke volume. The diastolic RV stroke volume can be measured by the 3D planimetered diastolic annular area multiplied by a PWD-derived velocity time integral (VTI) (22) (Figure 9B).
Congenital heart disease
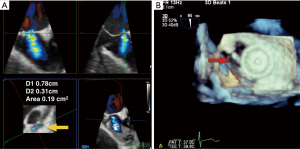
RT-3D TEE imaging is also used for transcatheter device closures of ASDs. Before a closure is attempted, 3D TEE assists in the 2D evaluation of the defect to determine size, shape, and relationship of the ASD to surrounding structures. During the percutaneous closure, 3D TEE is used to size the defect, usually by a single beat/multi beat narrow sector acquisition, with or without CFD, to determine both the short and long axis diameters of the defect in the anatomic and functional orifice (Figure 10A). A wider sector acquisition may be used to delineate the location of the defect with respect to its superior vena cava, inferior vena cava, coronary sinus and aortic borders. This allows the assessment of suitability of transcatheter closure. A rim of interatrial septal tissue (4 mm in each direction) is necessary between the defect and adjacent structures to seat the device (Figure 10B). Johri et al. tested the hypothesis that 3D TEE provides more accurate anatomic assessment of ASDs than 2D TEE. The authors found that while 3D TEE imaging properly identified ASD shape, and that maximal dimensions on 3D TEE were well correlated with balloon-sized 2D dimensions, 2D TEE underestimated these dimensions (23). Therefore, RT-3D TEE can be used to delineate the size, shape and number of defects better than 2D, and is particularly useful for assessment of multiple or irregularly shaped ASDs.
Left atrial appendage
Assessment of the LAA by 3D TEE has been well described, especially in excluding thrombus and guiding transcatheter LAA occlusion devices. The orthogonal imaging modality by matrix array 3D TEE probes (where two orthogonal planes are displayed simultaneously and where echocardiographer may adjust the imaging cursor plane to areas of interest within the LAA) can be helpful in excluding thrombus. For percutaneous LAA closure devices, 2D TEE measurement of ME view of LAA at 135° is presently the recommended method to size maximal left atrial appendage (LAA) orifice diameter. RT-3D TEE or gated acquisition may be helpful in sizing the orifice in different dimensions to more accurately determine a maximal orifice area, as well as guiding catheters during percutaneous procedures. Nucifora et al. assessed LAA measurements of 2D TEE and 3D TEE in comparison with CT measurements. Larger LAA diameters were measured using 3D TEE, versus 2D TEE (23.5±3.9 vs. 24.5±4.7 mm). 3D TEE measurements of LAA were not different from CT regarding number of lobes, area of orifice, and maximal diameter. When 2D TEE values were compared to 3D TEE and CT values, 3D TEE (24.5±4.7 mm) was more accurate in measuring maximal LAA diameter compared to 2D TEE (23.5±3.9 mm) (P<0.01). Therefore, the authors concluded that 3D TEE could be used to choose a more appropriate device size than 2D TEE (24,25).
Conclusions
Since 2010, RT-3D TEE use has increased inside and outside the operating room as an adjunct of the comprehensive 2D TEE exam. In fact, new 2013 ASE guidelines for comprehensive TEE recommend and include 3D focused acquisitions. However, due to the evolution of percutaneous cardiac interventions. Additionally, both RT-3D TEE and offline quantitative measurements from gated 3D acquisitions have become instrumental for procedural guidance, structural visualization of valves, ASDs, LAAs, and quantitative analysis of those structures. Due to continued evolution of percutaneous cardiac interventions, 3D imaging will likely impact the future use of perioperative TEE on a larger scale. With improved ultrasound quantification software, analysis of 3D acquisitions will become simplified. Automated RT-3D single beat full volume images obtained by new TEE probes with a high temporal resolution independent of motion artifacts, electrocautery, and arrhythmias will be the next major advance to facilitate 3D procedural imaging. With the increasing number of patients undergoing less invasive, percutaneous procedures, emerging-delete echocardiographers must be knowledgeable in 3D image acquisitions, familiar with the 3D anatomy of valves, able to communicate pathology effectively, and perform quantitative analysis of the various structural heart diseases encountered.
Acknowledgements
I would like to thank my senior colleague Dr. Adam David Lichtman for his great efforts in editing this manuscript.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Mahmood F, Shernan SK. Perioperative transoesophageal echocardiography: current status and future directions. Heart 2016;102:1159-67. [Crossref] [PubMed]
- Maxwell C, Konoske R, Mark J. Emerging Concepts in Transesophageal Echocardiography. F1000Res 2016;5. pii: F1000 Faculty Rev-340.
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Dorosz JL, Lezotte DC, Weitzenkamp DA, et al. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol 2012;59:1799-808. [Crossref] [PubMed]
- Meris A, Santambrogio L, Casso G, et al. Intraoperative three-dimensional versus two-dimensional echocardiography for left ventricular assessment. Anesth Analg 2014;118:711-20. [Crossref] [PubMed]
- Gaspar T, Adawi S, Sachner R, et al. Three-dimensional imaging of the left ventricular outflow tract: impact on aortic valve area estimation by the continuity equation. J Am Soc Echocardiogr 2012;25:749-57. [Crossref] [PubMed]
- Mahmood F, Matyal R. A quantitative approach to the intraoperative echocardiographic assessment of the mitral valve for repair. Anesth Analg 2015;121:34-58. [Crossref] [PubMed]
- Quader N, Davidson CJ, Rigolin VH. Percutaneous closure of perivalvular mitral regurgitation: how should the interventionalists and the echocardiographers communicate? J Am Soc Echocardiogr 2015;28:497-508. [Crossref] [PubMed]
- Cavalcante JL, Rodriguez LL, Kapadia S, et al. Role of echocardiography in percutaneous mitral valve interventions. JACC Cardiovasc Imaging 2012;5:733-46. [Crossref] [PubMed]
- Grewal J, Mankad S, Freeman WK, et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009;22:34-41. [Crossref] [PubMed]
- Manda J, Kesanolla SK, Hsuing MC, et al. Comparison of real time two-dimensional with live/real time three-dimensional transesophageal echocardiography in the evaluation of mitral valve prolapse and chordae rupture. Echocardiography 2008;25:1131-7. [Crossref] [PubMed]
- Wei J, Hsiung MC, Tsai SK, et al. The routine use of live three-dimensional transesophageal echocardiography in mitral valve surgery: clinical experience. Eur J Echocardiogr 2010;11:14-8. [Crossref] [PubMed]
- Biaggi P, Jedrzkiewicz S, Gruner C, et al. Quantification of mitral valve anatomy by three-dimensional transesophageal echocardiography in mitral valve prolapse predicts surgical anatomy and the complexity of mitral valve repair. J Am Soc Echocardiogr 2012;25:758-65. [Crossref] [PubMed]
- Cherry AD, Maxwell CD, Nicoara A. Intraoperative Evaluation of Mitral Stenosis by Transesophageal Echocardiography. Anesth Analg 2016;123:14-20. [Crossref] [PubMed]
- Guarracino F, Baldassarri R, Ferro B, et al. Transesophageal echocardiography during MitraClip® procedure. Anesth Analg 2014;118:1188-96. [Crossref] [PubMed]
- Shiota T. Role of echocardiography for catheter-based management of valvular heart disease. J Cardiol 2017;69:66-73. [Crossref] [PubMed]
- Franco E, Almería C, de Agustín JA, et al. Three-dimensional color Doppler transesophageal echocardiography for mitral paravalvular leak quantification and evaluation of percutaneous closure success. J Am Soc Echocardiogr 2014;27:1153-63. [Crossref] [PubMed]
- Saitoh T, Shiota M, Izumo M, et al. Comparison of left ventricular outflow geometry and aortic valve area in patients with aortic stenosis by 2-dimensional versus 3-dimensional echocardiography. Am J Cardiol 2012;109:1626-31. [Crossref] [PubMed]
- Jainandunsing JS, Mahmood F, Matyal R, et al. Impact of three-dimensional echocardiography on classification of the severity of aortic stenosis. Ann Thorac Surg 2013;96:1343-8. [Crossref] [PubMed]
- Khalique OK, Hamid NB, White JM, et al. Impact of Methodologic Differences in Three-Dimensional Echocardiographic Measurements of the Aortic Annulus Compared with Computed Tomographic Angiography Before Transcatheter Aortic Valve Replacement. J Am Soc Echocardiogr 2017;30:414-21. [Crossref] [PubMed]
- Hahn RT. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ Cardiovasc Imaging 2016;9:e005332. [Crossref] [PubMed]
- Johri AM, Rojas CA, El-Sherief A, et al. Imaging of atrial septal defects: echocardiography and CT correlation. Heart 2011;97:1441-53. [Crossref] [PubMed]
- Nucifora G, Faletra FF, Regoli F, et al. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circ Cardiovasc Imaging 2011;4:514-23. [Crossref] [PubMed]
- Yosefy C, Laish-Farkash A, Azhibekov Y, et al. A New Method for Direct Three-Dimensional Measurement of Left Atrial Appendage Dimensions during Transesophageal Echocardiography. Echocardiography 2016;33:69-76. [Crossref] [PubMed]


