Tailored antiplatelet therapy in high-risk ACS patients treated with PCI stenting: lessons from the ANTARCTIC trial
The recently reported ANTARCTIC trial by Cayla and colleagues is the latest large-scale study of personalized antiplatelet therapy based on platelet function testing (1). In this open-label, blinded end-point, randomized, controlled trial, the authors ascertained the net clinical benefit of individualized antiplatelet therapy based on platelet function monitoring in elderly patients undergoing stenting (PCI) for an acute coronary syndrome (ACS). Eight hundred and seventy-seven ACS patients aged over 75 years were randomly assigned to receive either prasugrel 5 mg daily with no monitoring or treatment adjustment (conventional group, n=442) or the same starting agent, but with the possibility to tailor antiplatelet therapy either through dose titration or switching to an alternative P2Y12 receptor inhibitor in case of inadequate platelet response (monitoring group, n=435). In the monitoring group, platelet function testing was assessed using the VerifyNowTM assay 14 days post-PCI and repeated 14 days later in patients who required therapy adjustment. High platelet reactivity (HPR) and low platelet reactivity (LPR) to adenosine diphosphate (ADP) were defined as >208 and <85 PRU, respectively. The dose of prasugrel was escalated from 5 to 10 mg daily in patients with HPR, or therapy was switched to clopidogrel 75 mg per day in patients with LPR.
The findings from this well-designed trial in elderly patients with ACS (who represent a population at high risk for ischemic and bleeding events) too frequently excluded from clinical studies, can be summarized as follows: (I) the platelet function testing guided-therapy approach resulted in treatment intensification for 4% of patients (16/435) and de-escalation of antiplatelet regimen for 39% (171/435) of patients in the monitoring group; (II) at 12 months, the primary composite end-point of cardiovascular death, myocardial infarction, stroke, definite stent thrombosis, urgent revascularization or bleeding, occurred in 120 (28%) patients in the monitoring group compared with 123 (28%) patients in conventional group (P=0.98); (III) the main safety endpoint of major bleeding (BARC type 2, 3 or 5) occurred in about a fifth of patients in each group (P=0.77); and (IV) this lack of statistical differences between groups was consistent across all the individual components of both ischemic and bleeding endpoints.
The ANTARCTIC trial is the latest in a string of trials reporting disappointing results for tailored antiplatelet therapy based on platelet function monitoring. Indeed, the rationale for personalized antiplatelet therapy stems naturally from numerous pharmacodynamic studies which demonstrated a wide variability of response to P2Y12 receptor inhibition in particular in patients treated by clopidogrel (2-4). Moreover, several reports suggested an association between HPR and post stenting ischemic events such as stent thrombosis or cardiovascular death (5), and a possible link between LPR and bleeding events in stented patients (6-8). Altogether, these observations led to the concept of a therapeutic window for DAPT (i.e., an “optimal” range of platelet reactivity) to improve outcomes, with the added complexity of defining standardized threshold values for HPR and LPR according to the type of platelet function assay used (9). However, so far studies on the potential of platelet function testing to improve outcomes in HPR patients undergoing PCI have yielded conflicting results in cohort studies and in randomized trials. Although promising results from smaller studies were reported (10), large-scale randomized, controlled trials consistently failed to establish any difference in cardiovascular outcomes between a conventional strategy and a platelet function monitoring-guided approach (Table 1). Some considerations help to position the ANTARCTIC trial results in the current landscape of platelet function-based tailoring of antiplatelet therapy.
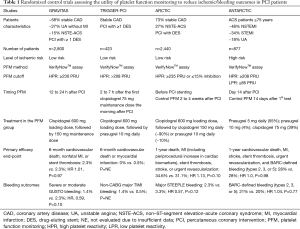
Full table
Previous studies have been criticized for enrolling low-risk patients whose rates of outcomes did not allow much differentiation between the standard-of-care and the adjusted treatment arms, as were the case in the GRAVITAS and the TRIGGER-PCI studies (11,12). In contrast, the ANTARCTIC trial targeted a higher risk population of patients, who indeed went on to have a higher rate of adverse events, but who nonetheless did not seem to benefit from tailored therapy. It has also been suggested that the therapeutic adjustments attempted provided only marginally altered platelet inhibition (11). While the intensification arm of ANTARCTIC did use an effective increase in prasugrel dose, it only occurred in 4% of patients receiving 5 mg of prasugrel daily. This rate is significantly lower than would have been expected, given that the GENERATIONS trial that assessed platelet function on prasugrel 5 vs. 10 mg in very elderly stable CAD patients, found 14% of patients presenting with HPR, with a more stringent HPR definition (13). It is therefore not clear why the ANTARCTIC study population, which should have presented with higher platelet reactivity due to their ACS presentation (14), had such an important underrepresentation of HPR leading to intensification of treatment. Other criticisms of prior trials included the fact that newer P2Y12 receptor inhibitors provide a significantly more predictable and profound inhibition of platelet function at the time of PCI than clopidogrel, advocating that these agents should be used at presentation to cover the HPR associated with ACS and PCI (11,12,15). The ANTARCTIC trial addressed this issue through homogeneous administration of prasugrel 5 mg to all participants, with possible de-escalation to clopidogrel 75 mg daily in patients with LPR. However, recent studies in elderly patients have shown similar rates of bleeding complications between patients treated with prasugrel 5 mg and clopidogrel 75 mg (13,16), and therefore the failure to improve the prognosis of patients in the monitoring group in the ANTRACTIC study might reflect the similar bleeding risks associated with these drug regimens rather than the actual predictive value of platelet function monitoring. The ongoing TROPICAL-ACS trial also includes a de-escalation arm based on platelet function monitoring, and should help shed light on this important issue (17). Finally, an important consideration that still plagues the field of personalized antiplatelet therapy, is the fact that all previous studies, including ANTARCTIC, investigated the question of a tailored antiplatelet therapy based on a single platelet function assay, the VerifyNowTM system (1,11,12,14). While the VerifyNowTM assay remains the most widely used and studied test for the link between HPR and clinical outcomes after PCI, other assays capture different aspects of platelet function and identify different patients as requiring antiplatelet therapy adjustment (18). Whether strategies based on alternative platelet function testing modalities would yield the same results remains an open debate, which the ongoing TROPICAL-ACS trial using the MultiplateTM technology may help to answer (17).
In summary, while the findings from the ANTARCTIC study are in line with previous trials of platelet function testing-driven personalization of antiplatelet therapy, there are still a number of open questions that require elucidation before recommendations for or against platelet function monitoring may be made. Moreover, it may be that a combination of clinical, genomic and pharmacodynamic variables will be necessary to provide the optimal patient profile to target personalized antiplatelet therapy. Ongoing research with alternative approaches to personalization is eagerly awaited.
Acknowledgements
Funding: M Lordkipanidzé is a Fonds de recherche du Québec - Santé (FRQS) Research Scholar (award number 33048).
Footnote
Conflicts of Interest: N Messas has received financial support (Fellowship grants) from Abbott Vascular France, Biotronik France and Biosensors France. JF Tanguay has received in-kind and financial support for physician-initiated grants from Spartan Bioscience Inc. (manufacturer of the Spartan RX CYP2C19), Roche Diagnostics (manufacturer of MultiplateTM), Aggredyne (manufacturer of AggreguideTM) and Eli Lilly Canada (manufacturer of prasugrel); received honorarium for speaker/consultation fees/advisory boards from AstraZeneca (manufacturer of ticagrelor) and Eli Lilly (manufacturer of prasugrel). M Lordkipanidzé has received in-kind and financial support for investigator-initiated grants from Roche Diagnostics (manufacturer of MultiplateTM) and Aggredyne (manufacturer of AggreguideTM).
References
- Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016;388:2015-22. [Crossref] [PubMed]
- Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908-13. [Crossref] [PubMed]
- Sibbing D, Schulz S, Braun S, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost 2010;8:250-6. [Crossref] [PubMed]
- Gurbel PA, Erlinge D, Ohman EM, et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA 2012;308:1785-94. [Crossref] [PubMed]
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919-33. [Crossref] [PubMed]
- Mokhtar OA, Lemesle G, Armero S, et al. Relationship between platelet reactivity inhibition and non-CABG related major bleeding in patients undergoing percutaneous coronary intervention. Thromb Res 2010;126:e147-9. [Crossref] [PubMed]
- Patti G, Pasceri V, Vizzi V, et al. Usefulness of platelet response to clopidogrel by point-of-care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty-Bleeding Study). Am J Cardiol 2011;107:995-1000. [Crossref] [PubMed]
- Tsukahara K, Kimura K, Morita S, et al. Impact of high-responsiveness to dual antiplatelet therapy on bleeding complications in patients receiving drug-eluting stents. Circ J 2010;74:679-85. [Crossref] [PubMed]
- Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62:2261-73. [Crossref] [PubMed]
- Aradi D, Komócsi A, Price MJ, et al. Efficacy and safety of intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after percutaneous coronary intervention: systematic review and meta-analysis. Int J Cardiol 2013;167:2140-8. [Crossref] [PubMed]
- Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097-105. [Crossref] [PubMed]
- Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol 2012;59:2159-64. [Crossref] [PubMed]
- Erlinge D, Gurbel PA, James S, et al. Prasugrel 5 mg in the very elderly attenuates platelet inhibition but maintains noninferiority to prasugrel 10 mg in nonelderly patients: the GENERATIONS trial, a pharmacodynamic and pharmacokinetic study in stable coronary artery disease patients. J Am Coll Cardiol 2013;62:577-83. [Crossref] [PubMed]
- Leé S, Vargová K, Hizoh I, et al. High on clopidogrel treatment platelet reactivity is frequent in acute and rare in elective stenting and can be functionally overcome by switch of therapy. Thromb Res 2014;133:257-64. [Crossref] [PubMed]
- Collet JP, Cuisset T, Rangé G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100-9. [Crossref] [PubMed]
- Erlinge D, Ten Berg J, Foley D, et al. Reduction in platelet reactivity with prasugrel 5 mg in low-body-weight patients is noninferior to prasugrel 10 mg in higher-body-weight patients: results from the FEATHER trial. J Am Coll Cardiol 2012;60:2032-40. [Crossref] [PubMed]
- Sibbing D, Aradi D, Jacobshagen C, et al. A randomised trial on platelet function-guided de-escalation of antiplatelet treatment in ACS patients undergoing PCI. Rationale and design of the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) Trial. Thromb Haemost 2017;117:188-95. [Crossref] [PubMed]
- Lordkipanidzé M. Platelet Function Tests. Semin Thromb Hemost 2016;42:258-67. [Crossref] [PubMed]


