PD-L1 protein expression in non-small cell lung cancer based on different immunohistochemical antibodies
Abnormal immune checkpoint activation leads to the immune escape of tumour cells, and one of the most important immune checkpoints is programmed cell death ligand 1 (PD-L1) (1). Our previous study showed that increased PD-L1 expression on tumour cells was significantly correlated with poor prognosis in breast cancer (2) gastric cancer (3), and non-small cell lung cancer (NSCLC) (unpublished results). However, the correlation between PD-L1 expression and prognosis remains controversial in NSCLC. Several studies demonstrated that high PD-L1 expression might be predictive of a poor prognosis. However, other studies could not confirm this finding (see Table 1). Whether the difference among these studies was the result of the use of different antibodies and cut-off values in each study is still unclear.
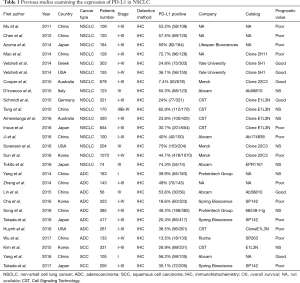
Full table
PD-L1 negatively regulates T-cell proliferation through the binding of programmed cell death protein 1 (PD-1) and induces activated T cell exhaustion and adaptive immune resistance (4). The blockade of the PD-1/PD-L1 pathway using monoclonal antibodies results in the restoration of activated T cells and is currently considered the most promising antitumour immunotherapy (5). A series of phase II-III studies have displayed the good clinical activity of PD-1/PD-L1 inhibitors in patients with NSCLC (6-9). However, an important issue is how to identify patients likely to benefit from PD-1/PD-L1 inhibitors. The findings of recent studies indicate that PD-L1 expression levels have emerged as a predictive biomarker useful for stratifying patients with NSCLC who are receiving PD-1/PD-L1 therapeutic agents (10). Each PD-1/PD-L1 inhibitor has been tested with a companion diagnostic assay or complementary diagnostic assay using different PD-L1 antibodies (clone 28-8, clone 22C3, clone SP142 and clone SP263), protocols, or cut-offs for PD-L1 positivity (see in Table 2). Therefore, it is imperative to compare the similarities and differences among 4 separate PD-L1 antibodies.

Full table
Recently, Hirsch et al. (11) reported the Blueprint PD-L1 Assay Comparison Project, which is collaboration between research organizations (the International Association for the Study of Lung Cancer and the American Association for Cancer Research), together with big pharma companies (Merck, Bristol-Myers Squibb, Genentech/Roche and AstraZeneca) and two diagnostic companies (Dako and Ventana). The Blueprint PD-L1 IHC Assay Comparison Project is planned in two phases. The aim of this project was to compare the performance of 4 PD-L1 IHC assays developed in combination with four PD-1/PD-L1 immune checkpoint inhibitors (Pembrolizumab, Nivolumab, Atezolizumab and Durvalumab) in NSCLC clinical trials. Four serial histologic sections from 38 NSCLC patients were stained with four PD-L1 IHC assays: 28-8 and 22C3 antibodies on the Dako Link 48 staining platform and SP142 and SP263 antibodies on the Ventana Benchmark platform. The slides were scanned and scored by three pathologists who estimated the percentages of tumour and immune cells that stained positive at any intensity. This study indicated that the percentage of PD-L1-stained tumour cells was comparable when the 22C3, 28-8, and SP263 assays were used, whereas the SP142 assay exhibited weak staining of tumour cell membranes. The concordance between four assays for immune cell staining appears to be lower than for tumour cell staining. It is recommended that the different trial-validated PD-L1 IHC assays should not be considered interchangeable. This is the first step in the harmonization of PD-L1 IHC assays and helps us to establish standardized and validated companion diagnostic tests. This study had several limitations. First, the number of patients enrolled in the study is relatively small. Second, PD-L1 expression was evaluated from NSCLC samples obtained by surgical resection in most cases. The consistency of surgically resected specimens and biopsy specimens remains unclear. An ongoing phase 2 of this study in a larger cohort of patients will assess agreements and discrepancies between surgically resected specimens and biopsy specimens. Third, it only compares the performance of four PD-L1 platforms; there are no therapeutic outcome data to evaluate the clinical predictive power of alternative PD-L1 IHC testing strategies.
Recent United States-based study concurs with the Blueprint study (12). This study was funded by pharmaceutical companies (Bristol-Myers Squibb) and the NCCN oncology research programme. It is a prospective, multi-centre, pathologist-based study. A total of 90 surgically resected samples of NSCLC were submitted to 4 PD-L1 IHC assays (clone 28-8, clone 22c3, clone SP142, and clone E1L3N). Compared with the Blueprint PD-L1 Assay Comparison Project, this study has more pathologists than any single assay. This study showed concordance between 3 of the 4 assays, and the SP142 assay was lower in staining intensity than the other 3 assays for both tumour proportion scores and immune cell proportion scores. This study also showed that high concordance among 4 separate PD-L1 antibodies for tumour cell staining and poor concordance for immune cell staining. This finding suggested that IHC may be a good way to detect PD-L1 expression in tumour cells but not in immune cells. Unfortunately, this study did not provide patients’ outcome data, and it could only evaluate diagnostic concordance and not clinical concordance. In 2017, Ratcliffe et al. (13) also analysed the concordance between three PD-L1 IHC diagnostic assays in patients with NSCLC (clone SP263, clone 22C3 and clone 28-8). This study included more samples than any other study. Four hundred and ninety-three patients with NSCLC were examined. The data showed that three PD-L1 IHC diagnostic assays had similar patterns of tumour membrane staining, with a high concordance rate among percentages PD-L1 staining. As a result of these studies with only diagnostic assays, the clinical utility of this assay needs to be verified in clinical studies.
In conclusion, this Blueprint study provided vital information regarding four diagnostic PD-L1 assays. Considering the limited number of patients in this study, the Blueprint results need to be validated in a larger, more comprehensive phase 2 study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Zhang M, Dong Y, Liu H, et al. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Sci Rep 2016;6:37933. [Crossref] [PubMed]
- Zhang M, Sun H, Zhao S, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 2017. [Epub ahead of print]. [PubMed]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [Crossref] [PubMed]
- Wang J, Yuan R, Song W, et al. PD-1, PD-L1 (B7-H1) and Tumor-Site Immune Modulation Therapy: the historical perspective. J Hematol Oncol 2017;10:34. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev 2015;41:868-76. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res 2017. [Epub ahead of print]. [Crossref] [PubMed]


