The effects of additional ezetimibe treatment to baseline rosuvastatin on circulating PCSK9 among patients with stable angina
Introduction
Cardiovascular disease (CVD) remains one of the commonest sources of morbidity and mortality in the world. Currently, coronary heart disease (CHD) is still the leading cause of cardiac death. Dislipidemia and especially high low density lipoprotein cholesterol (LDL-C) have contribute to the risk of CVD events, providing targets for clinical intervention. There are plentiful clinical trials about lipid management. The improvement of lipid metabolism, particularly for those who are estimated of high or extremely high risk, is believed to slow down atherosclerosis and reduce malignant cardiac events.
Lipid-lowering agents include bile acid sequestrants, nicotinic acids and ramification, 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMG CoA reductase), fibrates and ezetimibe. Moreover, new drugs such as acyl-coenzyme A-cholesterol acyltransferase (ACAT) inhibitor, microsomal triglyceride transfer protein (MTTP) inhibitor, proprotein convertase subtilisin kexin type 9 (PCSK9) monoclonal antibody are under clinical investigations. Statins, functioning as the HMG-CoA reductase inhibitor, not only regulate lipid metabolism, but also significantly decrease CVD events in the light of evidence-based medicine, thus remaining the cornerstone of intervention on lipids (1). Whereas, side effects such as liver or muscle harming (2,3) limit statins’ clinical use. Ezetimibe winning increasing attention in this field is known as the cholesterol absorption inhibitor with fewer adverse effects and higher safety than statins. The much-anticipated multicentral clinical trial—IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) has changed peoples’ insight towards ezetimibe. It conveyed that further LDL-C declining beyond that from optimized baseline statin therapy in patients with acute coronary syndrome (ACS) decreased non-fatal CVD events (32.7% vs. 34.7% CVD events at 7 years, P=0.02) (4). Meanwhile, no extra risk of cancer, muscle and gallbladder related events was found, verifying the safety of combination therapy of ezetimibe and statin.
PCSK9 is a protein recognized by investigators recently. It is excreted primarily from the liver, then combines with the low density lipoprotein receptor (LDL-R) on the hepatocyte surface to prompt LDL-R to degrade, hence upregulating the levels of circulating LDL-C (5). Activating mutations in PCSK9 give rise to a form of familial hypercholesterolaemia, while inactivating mutations lead to lower LDL-C levels and fewer CVD events (6). Therefore, to explore the impacts of diverse lipid-regulating agents on circulating PCSK9 appears highly imperative and indispensable. Existing investigations have unveiled the fact that statins may increase the levels of blood PCSK9 (7), impairing its efficacy in LDL-C reduction (8). Nevertheless, how PCSK9 responds to ezetimibe treatment has not been fully clarified (9,10). Naturally, how the combination therapy with a statin and ezetimibe wins stronger cardiac benefits via regulating PCSK9 becomes a point of interest. This study recruited patients with stable angina and aims to explore and clarify the short term impacts of rosuvastatin and ezetimibe, alone or in combination, on circulating PCSK9.
Methods
Study design and subjects
This study included a total of 68 and final 60 eligible patients with stable angina (4 did not meet the inclusion criteria, and 4 rejected to continue participating this trial after knowing the informed consent) in the Second Affiliated Hospital of Nanjing Medical University from December, 2014 to July, 2015. They were randomized to three treatment groups to receive a 14-day medication with either rosuvastatin 10 mg/d, ezetimibe 10 mg/d, or their combination. Human blood samples were obtained at baseline before treatment and at the 14th day after treatment to determine circulating LDL-C and PCSK9 levels. All participants confirmed the informed consents and this trial was approved by the ethics committee of the Second Affiliated Hospital of Nanjing Medical University (No. 2014KY044). Inclusion criteria (8) were stable angina patients diagnosed (11) within 5 years, age between 18 and 70 years, body mass index (BMI) between 18.5 and 30 kg/m2, normal blood pressure (<140/90 mmHg). Exclusion criteria were subjects who had received lipid-lowering drugs within 4 weeks prior to study entry, those with a history of excessive alcohol intake, liver disease, renal dysfunction (glomerular filtration rate <60 mL/min), rheumatologic disease, diabetes or other endocrine disorders, history of recent substantial (>10%) weight change, history of obesity (BMI >35 kg/m2), or taking medications known to affect lipoprotein metabolism, glucose metabolism, or the immune system.
Measurement
Blood concentrations of LDL-C were measured by enzymatic methods. Circulating PCSK9 concentrations were determined by sensitive and specific sandwich ELISA assay. The microtiter plate provided in the human PCSK9 ELISA kit (CUSABIO, Wuhan, China) had been pre-coated with PCSK9. 50 µL human serum samples were then added to the appropriate microtiter plate wells with 50 µL horseradish peroxidase (HRP) conjugated antibody preparation specific for PCSK9. Incubation continued for 30 minutes at 37 °C, and then aspirated the fluid and washed for 5 minutes. The competitive inhibition reaction was launched between pre-coated PCSK9 and PCSK9 in samples. 90 µL substrate solution was added to each well and incubation continued for 20 minutes at 37 °C. The color developed in opposite to the amount of PCSK9 in the samples. Once the color development was stopped after adding 50 µL stop solution to each well, the intensity of the color was measured at 450 nm within 5 minutes.
Statistical analyses
All descriptive data were given as mean ± standard deviation or proportions (in percent). The Sapiro-Wilk test was used to observe whether all data were subject to normal distribution. If the quantitative data were in normal distribution and had the homogeneity of variance, Student-t test or ANOVA test was used. All numeration data were analyzed by the chi square test. Changes of PCSK9 or LDL-C in the same group between baseline and endpoint were compared by paired t test. Bivariate correlations were examined using Spearman’s rank test to identify which parameters influence the change of PCSK9, and a multiple regression analysis was then performed. P<0.05 was considered to have statistical significance. All statistical analyses were done using SPSS18.0 software.
Results
This study ultimately included 60 eligible patients with stable angina, including 36 males and 24 females. Within the total cohort, the general information representing the basic characteristics of patients that may influence the levels of circulating PCSK9 (10) statistically was found with no significant difference at baseline among groups, neither in age, BMI, sex, smoking, total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), LDL-C or PCSK9 (Table 1).
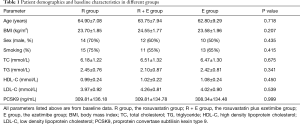
Full table
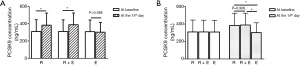
At the 14th day of the experiment, blood PCSK9 levels and the concentrations of different lipids were measured (Table 2) and contrastive analysis was taken between different time points. Figure 1 shows the changes of PCSK9 levels in the three groups at baseline and the endpoint of the treatment. Compared with the baseline data prior to treatment, the circulating PCSK9 levels in the rosuvastatin-treated group were increased, with statistical significance (309.81±136.18 vs. 383.30±139.87 ng/mL, P<0.05) (Figure 1A). The levels of circulating PCSK9 in the rosuvastatin plus ezetimibe group were also found significantly higher than their couterparts at baseline after 2-week medication (309.81±134.78 vs. 388.50±135.68 ng/mL, P<0.05) (Figure 1A). But as regards to the ezetimibe mono-drug treatment, the increment of PCSK9 did not show statistical significance between pre- and post-intervention (308.34±134.48 vs. 301.99±112.58 ng/mL, P=0.558) (Figure 1A). At the 14th day of treatment (Figure 1B), no significant difference was found between the rosuvastatin and the combination groups in circulating PCSK9 levels (383.30±139.87 vs. 388.50±135.68 ng/mL, P=0.906). However, both the above two groups were with higher levels of PCSK9 than the ezetimibe group (rosuvastatin versus ezetimibe, 383.30±139.87 vs. 301.99±112.58 ng/mL, P=0.05; rosuvastatin plus ezetimibe vs. ezetimibe, 388.50±135.68 vs. 301.99±112.58 ng/mL, P<0.05). As shown in Figure 1, all the three groups shared parallel levels of circulating PCSK9 at baseline. However, either rosuvastatin or its combination with ezetimibe, other than ezetimibe mono-therapy, was supposed to raise circulating PCSK9. Moreover, rosuvastatin treatment or its co-administration with ezetimibe showed parallel effects on regulating PCSK9 (percent enhancement of PCSK9, 31.50%±20.11% vs. 34.80%±26.38%, P=0.659).
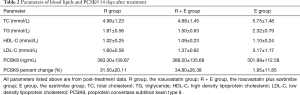
Full table
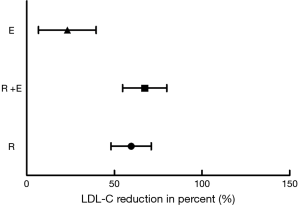
The changes of blood LDL-C levels at baseline and the treatment end-point respectively in the three investigated groups are conveyed in Figure 2. Similarly, rosuvastatin, ezetimibe or they together were all found to lower the levels of circulating LDL-C. Either rosuvastatin or its combination with ezetimibe had the potential to bring about a sharper decrease in LDL-C concentrations, rather than ezetimibe mono-treatment (rosuvastatin vs. ezetimibe, 59.60%±11.46% vs. 23.18%±16.43%, P<0.001; rosuvastatin plus ezetimibe vs. ezetimibe, 67.33%±12.59% vs. 23.18%±16.43%, P<0.001). Above all, when rosuvastatin was given together with ezetimibe, circulating LDL-C levels declined more sharply than those under rosuvastatin single-drug therapy (rosuvastatin versus rosuvastatin plus ezetimibe, 59.60%±11.46% vs. 67.33%±12.59%, P=0.049).
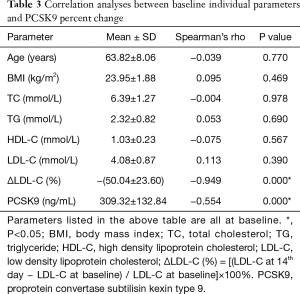
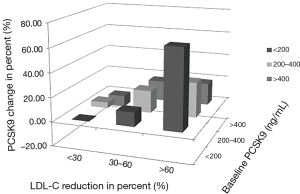
The augment in PCSK9 (expressed as percent change) was initially discovered correlated with baseline PCSK9 levels (Spearman rho =−0.554, P<0.001) and with the percent reduction of LDL-C concentrations (Spearman rho =0.949, P<0.001) by bivariate correlation analysis (Table 3). The above two factors were then recruited in multiple linear regression analysis for further exploration. According to the final fit model, both the percent reduction of LDL-C from baseline (β1=0.007, t=10.095, P<0.001) and baseline PCSK9 levels (β2=−0.001, t=−4.577, P<0.001) showed significant influences on PCSK9 concentration changes in percent. This result explained a substantial and significant proportion of the variance in the change of PCSK9 (R2=0.802, F=115.079, P<0.001). As shown in Figure 3, the most dramatic increase in circulating PCSK9 was found in the column with low PCSK9 concentrations (<200 ng/mL) at baseline and profound LDL-C reductions (≥60% from baseline). In columns with weak LDL-C reductions (<30% from baseline), PCSK9 concentrations were hardly affected (−10% to +5%) whatever the baseline PCSK9 levels were. Moderate LDL-C reduction in percent (30% to 60%) caused obvious PCSK9 upregulation (12% to 19%). But when LDL-C declined robustly (≥60%), the percent promotion of PCSK9 sharply occurred and varied from 19% to 66%, reaching a summit effect in column with the lowest baseline PCSK9 concentrations.
Full table
Discussion
Statins, inhibiting cholesterol synthesis, are commonly chose agents for lipid-lowering, and have the potential to reduce LDL-C by 40–60%. Ezetimibe acts as an inhibitor of cholesterol absorption, decreasing LDL-C by 20% or so. Statin and ezetimibe are often administered together clinically, to realize a higher lipid drop (12). PCSK9 is closely correlated with cholesterol metabolism. It combines with epidermal growth factor A (EGF-A) on LDL-R, forming PCSK9-EGF-A complexes which increase the degradation of the LDL-R by downregulating its utilization, thus increasing the blood LDL-C concentrations (5,13-15). PCSK9 concentrations have been regarded to be associated with hypercholesterolaemia, major CVD events or even mortalities and morbidities (6,16,17). According to previous investigations, statins could upregulate the levels of LDL-R and PCSK9 (7). In this point of view, the increment in PCSK9 concentration may impair benefits from statins for patients (8,18). Consequently, additional strategies for optimized baseline statin therapy seem imperative and indispensable (9).
Our current randomized trial investigated the effects of two lipid-regulating agents rosuvastatin and ezetimibe on PCSK9 concentrations, alone and in combination. The major findings were as follows: (I) rosuvastatin could not only lower the levels of LDL-C, but also yield increase of circulating PCSK9; (II) however, while lowering LDL-C, ezetimibe did not result in any significant changes in blood PCSK9 concentrations; (III) ezetimibe additional to rosuvastatin brought about more extensive LDL-C drop but no significant elevation in circulating PCSK9 when compared to rosuvastatin mono-treatment. As additional ezetimibe to rosuvastatin did not increase blood PCSK9 further, therefore, no greater harm was done to rosuvastatin single therapy. These results are in accordance with Berthold’s findings (10). A large scale research by Chang SH and his team showed that simvastatin combined with ezetimibe caused lower incidence of CVD events in patients with type 2 diabetes than simvastatin mono-drug intervention (19). Besides, The notable double-blinded, randomized and controlled multicenter trial—IMPROVE-IT, including 18,144 patients with ACS, has brought the following exciting reports: ezetimibe added to baseline simvastatin therapy can moderately reduce CVD events, obtaining further clinical benefits (4). In consistence, our study suggests a probable mechanism that the use of statin drugs plus ezetimibe treatment is likely to realize sharper reduction in LDL-C levels, but little influences blood PCSK9, thus maintaining maximal lipid-drop and cardioprotection benefits. As a result, when the maximally tolerated dose of statin therapy fails to achieve the target LDL-C, it needs to consider alternative treatment options after statin use. In these cases, combination therapy should be considered (20).
Potential factors that may impact circulating PCSK9 levels have been further explored in this study by bivariate correlation and multiple linear regression analyses. Finally, the percent change of PCSK9 is found closely correlated with LDL-C reduction in percent from baseline and the PCSK9 levels at baseline. Reviewing the coefficients’ absolute value in the regression equation, the factor LDL-C percent reduction contributes more than the baseline PCSK9. Besides, the baseline PCSK9 plays a protective role while the LDL-C percent reduction acts as a risk factor. It can be visualized in Figure 3 that a weak decline in LDL-C only contributes to tiny PCSK9 upregulation. This situation lasts until LDL-C reduction becomes moderate or even remarkable. Consequently, ezetimibe shows weak interactions towards PCSK9 changes because of its unsubstantial effects in LDL-C reduction, despite the basal levels of PCSK9. Rosuvastatin therapy (alone or in combination) is responsible for substantial LDL-C reduction, the latter evoking circulating PCSK9 elevation. Actually, we observed no significant circulating PCSK9 change after additional ezetimibe administration to baseline rosuvastatin treatment; although a further decline in LDL-C level appeared. It can be speculated that percent change of PCSK9 merely varies linearly with LDL-C reduction within a specific interval. Once the LDL-lowering medication results in a LDL drop exceeding the interval, little room is still left for circulating PCSK9 to go up to a significant extent. There may exist a plateau or hyporesponsive period for PCSK9 when answering to different lipid lowering strategies. In this study, administration with 10 mg rosuvastatin daily for 14 days sufficiently raises circulating PCSK9 concentrations to a plateau, leaving little room for incremental PCSK9 promotion from further LDL-C lowering. This plateau effect has also been confirmed by many other investigators when compared between different doses of statins (16). Besides the mechanism mentioned above, statins may upregulate PCSK9 concentrations by peroxisome proliferator-activated receptor (PPAR) related pathway (21,22) or sterol regulatory element binding protein-2 (SREBP-2) associated mechanism (7,23-25). However, ezetimibe has not been discovered with such pleiotropic effects. This could explain from another perspective that why additional PCSK9 increase does not appear along with further LDL-C drop by ezetimibe when added to rosuvastatin.
Limitations
Although our study intuitively reflects the real conditions among patients with stable angina, yet it still has limitations. First, the relatively small scale of our research may lead to certain underestimation or loss of data associations, which may rewrite the multiple regression analysis and subsequent interpretation. Second, the research duration was not long enough to observe mid or long-term results, although longer lasting investigations unveiling parallel outcomes have demonstrated that the maximal lipid-lowering effect of statins and ezetimibe appears within 2 weeks (26-28). Third, rosuvastatin we chose in this study could not represent other kinds of statins. Whether the combination effects of these statins with ezetimibe shared the analogical properties still needed further clarification and illumination.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Scientific Research Ethics Review Committee of the Second Affiliated Hospital of Nanjing Medical University (No. 2014KY044), and all subjects signed a written informed consent form.
References
- Mirjanic-Azaric B, Rizzo M, Jürgens G, et al. Atorvastatin treatment increases plasma bilirubin but not HMOX1 expression in stable angina patients. Scand J Clin Lab Invest 2015;75:382-9. [Crossref] [PubMed]
- Jiménez-Navarro MF, López-Jiménez F, Barsness G, et al. Long-term prognosis of complete percutaneous coronary revascularisation in patients with diabetes with multivessel disease. Heart 2015;101:1233-9. [Crossref] [PubMed]
- Shirokova S, Ilashchuk T, Okipniak I. The comparative effectiveness of beta-blockers and inhibitors of if-iodium using in patients with stable angina pectoris. Georgian Med News 2015.25-8. [PubMed]
- Špinar J, Špinarová L, Vítovec J. IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (studie IMPROVE-IT). Vnitr Lek 2014;60:1095-101. [PubMed]
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 2012;11:367-83. [Crossref] [PubMed]
- Wierzbicki AS, Grant P. Drugs for hypercholesterolaemia - from statins to pro-protein convertase subtilisin kexin 9 (PCSK9) inhibition. Clin Med (Lond) 2016;16:353-7. [Crossref] [PubMed]
- Tibolla G, Norata GD, Artali R, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): from structure-function relation to therapeutic inhibition. Nutr Metab Cardiovasc Dis 2011;21:835-43. [Crossref] [PubMed]
- Seidah NG. PCSK9 as a therapeutic target of dyslipidemia. Expert Opin Ther Targets 2009;13:19-28. [Crossref] [PubMed]
- Konrad RJ, Troutt JS, Cao G. Effects of currently prescribed LDL-C-lowering drugs on PCSK9 and implications for the next generation of LDL-C-lowering agents. Lipids Health Dis 2011;10:38. [Crossref] [PubMed]
- Berthold HK, Seidah NG, Benjannet S, et al. Evidence from a randomized trial that simvastatin, but not ezetimibe, upregulates circulating PCSK9 levels. PLoS One 2013;8:e60095. [Crossref] [PubMed]
- Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006;27:1341-81. [Crossref] [PubMed]
- Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008;198:198-207. [Crossref] [PubMed]
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009;50 Suppl:S172-7. [Crossref] [PubMed]
- Qian YW, Schmidt RJ, Zhang Y, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res 2007;48:1488-98. [Crossref] [PubMed]
- Zhang DW, Lagace TA, Garuti R, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem 2007;282:18602-12. [Crossref] [PubMed]
- Huijgen R, Boekholdt SM, Arsenault BJ, et al. Plasma PCSK9 levels and clinical outcomes in the TNT (Treating to New Targets) trial: a nested case-control study. J Am Coll Cardiol 2012;59:1778-84. [Crossref] [PubMed]
- Vlachopoulos C, Terentes-Printzios D, Georgiopoulos G, et al. Prediction of cardiovascular events with levels of proprotein convertase subtilisin/kexin type 9: A systematic review and meta-analysis. Atherosclerosis 2016;252:50-60. [Crossref] [PubMed]
- Careskey HE, Davis RA, Alborn WE, et al. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res 2008;49:394-8. [Crossref] [PubMed]
- Chang SH, Wu LS, Lee CH, et al. Simvastatin-ezetimibe combination therapy is associated with a lower rate of major adverse cardiac events in type 2 diabetics than high potency statins alone: A population-based dynamic cohort study. Int J Cardiol 2015;190:20-5. [Crossref] [PubMed]
- Krähenbühl S, Pavik-Mezzour I, von Eckardstein A, et al. Unmet Needs in LDL-C Lowering: When Statins Won't Do! Drugs 2016;76:1175-90. [Crossref] [PubMed]
- Han L, Li M, Liu Y, et al. Atorvastatin may delay cardiac aging by upregulating peroxisome proliferator-activated receptors in rats. Pharmacology 2012;89:74-82. [Crossref] [PubMed]
- Sanderson LM, Boekschoten MV, Desvergne B, et al. Transcriptional profiling reveals divergent roles of PPARalpha and PPARbeta/delta in regulation of gene expression in mouse liver. Physiol Genomics 2010;41:42-52. [Crossref] [PubMed]
- Dubuc G, Chamberland A, Wassef H, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2004;24:1454-9. [Crossref] [PubMed]
- Rashid S, Curtis DE, Garuti R, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci U S A 2005;102:5374-9. [Crossref] [PubMed]
- Berge KE, Ose L, Leren TP. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler Thromb Vasc Biol 2006;26:1094-100. [Crossref] [PubMed]
- Knapp HH, Schrott H, Ma P, et al. Efficacy and safety of combination simvastatin and colesevelam in patients with primary hypercholesterolemia. Am J Med 2001;110:352-60. [Crossref] [PubMed]
- Bays HE, Moore PB, Drehobl MA, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther 2001;23:1209-30. [Crossref] [PubMed]
- Lakoski SG, Xu F, Vega GL, et al. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. J Clin Endocrinol Metab 2010;95:800-9. [Crossref] [PubMed]


