Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)—from morphology to molecular testing
Introduction
The traditional approach to the diagnosis of lung and mediastinal diseases has included different noninvasive and invasive techniques performed to obtain diagnostic cytological and/or histological material. Among invasive techniques, aspiration cytology including transbronchial needle aspiration (TBNA) and computed tomography (CT)-guided transcutaneous fine needle aspiration/core biopsy have been used for many decades as an alternative to more invasive surgical procedures. However, in recent years, endobronchial ultrasound-guided TBNA (EBUS-TBNA) has emerged as an innovative technique and has been successfully introduced into daily clinical practice (1). This procedure has joined the use of conventional bronchoscopy and ultrasound imaging, which are two techniques well managed for decades, with needle aspiration beyond the bronchial wall to get material from both lung parenchyma and mediastinal lymph nodes. Indeed, this procedure is of particular interest either to confirm the diagnosis of radiologically detected central lung cancer, or for the staging of known lung cancer by sampling mediastinal lymph nodes. Moreover, EBUS-TBNA can be particularly useful for the diagnosis of localized mediastinal disease or other lung diseases associated with mediastinal lymphadenopathy like sarcoidosis (2).
The results just obtained after the introduction of this novel technique in terms of diagnostic accuracy and safety has convinced clinicians that it may represent an innovative diagnostic tool with several advantages including minimally invasive approach, safe, cost-effective, real time image guidance, broad sampling capability, and rapid on-site evaluation (ROSE) (3,4). EBUS-TBNA has also acquired importance in cases in which surgical procedures are contraindicated or unnecessary, like patients with co-morbidity which increase surgical risks, and it is more suitable for patients with disease not requiring surgery such as lymphomas, germ cell neoplasms or metastatic lung cancers.
Using the EBUS-TBNA approach it is possible to obtain either cytological material (with or without ROSE) or histological cores. Although literature data comparing the diagnostic accuracy between the two material typologies are limited, available information has not demonstrated a significant difference. A perspective study comparing EBUS-TBNA with EBUS biopsy without aspiration has not demonstrated differences in sample quality and diagnostic yield for malignancy (5). Consequently, the choice of the type of material (cytological versus histological) mainly depends on the local expertise and organization.
In this study we give a review on the practical approach to EBUS-TBNA procedure including cytological, histological, and molecular aspects. The new era of EBUS-TBNA may be resumed as “do more with less” because with a relatively small material we are now able to perform an accurate diagnosis associated with prognostic and predictive morphological and molecular markers.
Cytology approach to EBUS-TBNA specimens
Considering the cytological approach to EBUS-TBNA specimens, sensitivity in the diagnosis of lung cancer is recorded to be up to 90% after learning curve (4) and diagnostic accuracy for this technique is reported to be higher than that for CT and PET (98% vs. 60.8% and 72.5%, respectively). The benefit of ROSE during EBUS-TBNA has been matter of debate in the last years. Indeed, although some studies concluded that ROSE does not increase the diagnostic efficacy of EBUS-TBNA (6), other several investigations have demonstrated that it allows to improve the diagnostic yield of the procedure up to 30% (7-9) and that the immediate evaluation of sample adequacy allows to avoid additional biopsies, additional bronchoscopic procedures, repetition of other diagnostic procedures or risk of complications connected to bronchoscopy (10). Furthermore the sample preparation by cytopathologist is optimized with the aid of direct macroscopic inspection, optimal smearing techniques, and triage of the sample allowing to obtain adequate tissue for diagnosis, ancillary techniques and molecular testing (7).
For this reason, the cytopathologist is more and more solicited to offer ROSE evaluation of cytological material obtained during EBUS-guided technique and has assumed an increasingly central role in the management of patients with lung cancer. In situations where financial difficulties do not permit to have enough resources, the cytotechnologists (CyT) may also be asked to give rapid interpretations. The accuracy rate of responses done by CyT (between preliminary and final diagnosis) was showed to be higher for pancreas, liver and bone lesions and lower for kidney and lung lesions (11).
There is a lot of variability in technical methods for tissue acquisition and further treatment of collected specimens. Variability concerns necessity to have ROSE, needle gauge, number of passes and specimens’ management. ROSE is essential for appropriateness of tissue management, as abundance of material is now the goal of image-guided procedures for targeted therapies. Moreover, with ROSE, TurnAround Time (the amount of time taken to give a diagnosis, TAT) is reduced. Most studies showed a 90% to 98% concordance between ROSE diagnosis and final diagnoses (8,12). False negative rate due to sampling errors is directly correlated to the lower number of slides examined. EBUS-TBNA is also frequently performed because of the discovery of a PET positive lesion/nodule in patients followed up for previous lung malignancies. In this situation, it has been demonstrated that ROSE significantly reduces false positive rate in PET positive lesions.
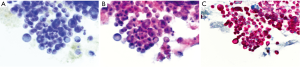
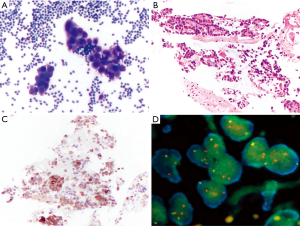
Usually, needle gauge used is 22. In our experience, the number of passes is determined by the acquisition of diagnostic material. In case of positive (malignant) diagnosis on one of the ROSE slides, we try to have an idea of the possible diagnosis/differential diagnosis. Thus, we can triage the material appropriately according to the preliminary working diagnosis: cell block preparation in case of solid tumor for immunocytochemistry and mutational analysis, flow cytometry in case of suspected hematological malignancies, and sterile sampling for microbiological studies in case of inflammatory process/abscessed cavity. The rest of the passes will be used to enrich one of these possibilities. In case of negative (normal tissue) diagnosis, such as in case of reactive lymph nodes, the needle rinse material after each pass will be used for liquid-based cytological preparation. Three passes are generally sufficient to provide enough material. After each pass, one to three slides can be realized and stained with Toluidine blue (Figure 1A). The staining process lasts no more than 10–15 seconds and permits a rapid, almost immediate, response from the cytopathologist or the trained CyT. What should we communicate to the clinicians? Strictly, the most appropriate rapid diagnosis concerns the quantity of material aspired: sufficient (or not) to establish a definitive diagnosis. However, according to the confidence between cytopathologists and clinicians, and also according to the compliance of the clinicians to understand and accept that definitive diagnosis may change the preliminary one, the cytopathologists can also say more: material consistent with a lymph node tissue (thus indicating the appropriateness of the technical aspiration) or consistent with malignant cells (i.e., adenocarcinoma vs. squamous cell carcinoma vs. small cell carcinoma). This is particularly useful in urgent cases in which a diagnosis of small cell neuroendocrine carcinoma can immediately prompt the clinicians towards a chemotherapeutical decision, instead of a surgical treatment. Once the diagnosis is reached on one smear, all the rest of the material is used to enrich the specimens or for special tests. Once in the cytopathology laboratory, the Toluidine blu smear is then stained with traditional Pap stain for final interpretation (Figure 1B) and the rest of the material is treated for liquid-based cytology or cell block preparations. Immunocytochemical stains (Figure 1C) and molecular analysis can be done using either stained slides or cell block.
Histological approach to EBUS-TBNA specimens
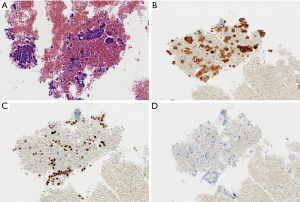
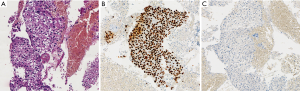
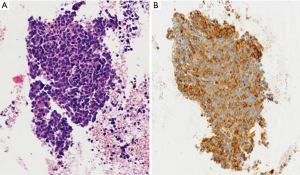
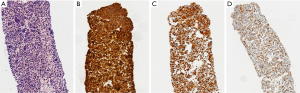
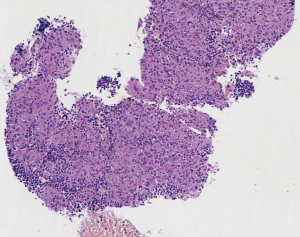
EBUS biopsy without aspiration has been referred as “transbronchial needle capillary sampling (TBNCS)” by some authors (5). Since it seems that there is not a significant difference in diagnostic yield between EBUS-TBNA and TBNCS, the choice of the type of material (cytological vs. histological) mainly depends on the local expertise and organization. The disadvantage in using histological material mainly relies on the impossibility to have a rapid on site evaluation. Conversely, the advantage relies on the possibility to have more material for immunohistochemical and molecular analysis which may be very important for certain tumor types including lymphomas and adenocarcinomas (Figures 2-5). In addition, lymph node biopsy may be better in getting material than cytological approach when lymph node is fibrotic such as in cases of sarcoidosis (Figure 6). From a practical point of view, the material is routinely managed as a histological core biopsy. In our experience, cytological examination of cell block obtained from needle washing material is helpful to get additional diagnostic cells.
Molecular testing
The vast majority of patients with non-small cells lung carcinomas (NSCLC) does not require surgical resection because of the advanced stage at clinical onset. For this reason, the unique specimen available for these patients is frequently represented by small cytological or micro-histological biopsy samples. On the other hand, an accurate and precise diagnosis (i.e., recognition of benign vs. malignant, primary vs. metastatic lesion and the NSCLC subtyping), together with the determination of predictive biomarkers, are mandatory for treatment selection. In this scenario, the major issue is to maximize all necessary information on cytological or biopsy specimen available.
Although performing diagnoses on small samples could be easy (in case of morphology-based adeno or squamous cell carcinoma features), in several cases of poorly differentiated or undifferentiated cancers morphology does not allow a more precise classification and the discouraged term NSCLC-not otherwise specified (NOS) is used. However, major therapeutic advances in lung cancer field require a more precise histological subtyping for therapeutic purposes (13,14). The use of immunohistochemistry (IHC) in these cases is recommended because may add some informations about a residual adeno- or squamous differentiation, increasing the refinement of diagnosis and avoiding the use of NSCLC-NOS term (15). Several biomarkers and diagnostic algorithms have been described in the last years and suggested for the most efficient NSCLC subtyping: nowadays, using a small panel of 2 to 4 biomarkers (i.e., TTF1, Napsin A, p40 and CK5/6 or CK7) the proportion of NSCLC-NOS diagnosis could be widely reduced (15,16). This is an important aim, since morphologically poorly differentiated carcinomas with an adenocarcinoma immunoprofile show the same overall survival of cases with clear morphological features of adenocarcinoma (17,18). Furthermore, the more and more precise molecular profiling of lung cancer has led to the discovering of an increasing number of new driver genes that could become drug target (19). Lung adenocarcinoma is the most studied in this direction because it is characterized by a highly druggable molecular profile. In fact, an increasing number of genes are described as oncogenic drivers and several drugs are developed for each of them (19). As a matter of fact, patients with an oncogenic driver mutation who receive targeted therapy have better survival than patients who do not receive targeted therapy or without an oncogenic driver (20). However, after a honeymoon period of response to therapy, acquired resistances can occur (21). The vast majority of cancers treated with target therapy harbor therapy-induced new genetic changes, in term of both acquisition and loss of gene alterations (22,23). Moreover, histological shift from well differentiated to poorly differentiated small cell cancer has been described as acquired resistance mechanism (23-25). Comprehensive molecular profiling of other more frequent lung cancer types including squamous and large cell carcinoma revealed a marked genomic complexity with less druggable alterations for the first and genetic features typical of other better differentiated carcinomas for the latter (26,27). Finally, with the advent of the target therapy, a new predictive role for IHC has been awarded. Indeed, IHC allows to visualize the presence and tissue distribution of the drug target at the protein level. For example in ALK test, the gene alteration produces a fusion protein which is easily detectable by IHC. To this end two monoclonal antibodies have been developed and are commercially available: the clone 5A4 (Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, UK/Novocastra Laboratories Ltd., Newcastle Upon Tyne, UK, and prediluted Abcam, Cambridge, UK), and the clone D5F3 (Cell Signaling Technology Inc., Danvers, MA, USA) with high sensitivity, specificity and reproducibility, as compared to FISH technique. That’s why the use of ALK IHC as screening test has been adopted in some countries and only tumors that are positive for ALK IHC, either weakly or strongly, should be referred to FISH for confirmation of a rearrangement (28).
A field in great expansion is the use of immunotherapy in combination with conventional therapy (29). Immune check-point is the receptor-ligand interaction that leads to a modulation of the tumor-induced immune response. The PD-1/PD-L1 interaction is one of the more widely studied because PD-L1 IHC expression on tumor cells has been associated with higher response rate to anti-PD-1/PD-L1 drugs, although some methodological issues have still to be resolved (30), such as the percentage of positive cells to be considered predictive of a satisfactory clinical response and the type of the primary antibody used.
In the last WHO 2015 (15), specific recommendations for pathologists about lung cancer classification in small tissue together with an algorithm for handling samples have been published, including three major steps: the accurate diagnosis, the use of IHC for poorly differentiated NSCLC and the requirement of mutational assay. These guidelines imply that the tissue represents the major issue in advanced lung cancer pathology, with some technical aspects to keep in mind for pathologists: first of all the sample evaluation. Pathologist should be present during the sampling procedures to collect clinical information, to handle and prepare tissue sampling for a rapid onsite staining and ROSE which provides a real time evaluation of cytological specimen obtained with cytological sampling (see above). The use of ROSE permits to obtain sufficient tissue for molecular testing with high rate of success (31,32); in fact, although actually there is no evidence to recommend the use of ROSE in all the procedures, guidelines suggest that ROSE should be used when molecular testing is needed (12). A recent randomized trial study by Trisolini and coworkers showed that ROSE is associated with a 10% increase in the success rate of EBUS-TBNA for optimal lung cancer genotyping, with the advantage of reducing the number of re-biopsy because of the minimal tumor burden for molecular analysis (33). Finally, when the sample is processed according to routine management, the final sample should be evaluated for the assessment of the amount and quality of the neoplastic cells, necessary for the type and the number of molecular analysis required. At this step, a crucial issue is the choice of the marker to test, the choice of the optimal method to use and the interpretation of results according to guidelines. Nowadays, EGFR mutations and ALK translocation are the most predictive tests required for targeted therapies (34,35), but several other gene mutations (i.e., KRAS, BRAF, etc.) or alterations (i.e., ROS translocation, MET amplification, etc.), predictive of response or resistance to therapy, are also increasing in importance. According to specific gene alterations (mutation, translocation or amplification) a specific method should be used and appropriate tissue amount and quality is required. For example, EGFR mutation test requires an adequate amount of neoplastic DNA with respect normal DNA to maximize the sensitivity of the sequencing method used (i.e., direct sequencing, pyrosequencing, etc. Figure 7) and for this reason a sample with an adequate percentage of neoplastic cell with respect to the normal counterpart is necessary. Cytological samples are generally characterized by few neoplastic cells in a background of normal cells: these few cells could be sufficient for a diagnosis of malignancy but not adequate for molecular analyses. To improve the availability and adequacy of cytological material and to avoid the poor neoplastic cellularity of certain cytological samples, an accurate microscopic manual microdissection could be performed in the cell block, smear or core biopsy and multiple sections from cell block could be used to increase the amount of extracted DNA. On the contrary, for ALK translocation assessment, a minimum of 50 neoplastic cells should be present in the same cut section according to the cut-off guideline of ALK translocation assessment based on 50 counted nuclei (Figure 8).

Recent guidelines (12) in the material acquisition and preparation to obtain adequate EBUS-TBNA specimens recommend as follows: (I) at least three needle aspirations per site for morphological diagnosis and a total of four passes per site when molecular testing is planned should be performed; (II) preservation of material for production of cell blocks or tissue core for morphological evaluation and for IHC for subtyping NSCLC, when is needed, should be planned. There is no quality evidence to suggest the best solution (e.g., formalin, saline or Hank’s solution) for cell block preparation and it should be chosen following consultation with local and molecular pathology colleagues; furthermore, no specific slide preparation and staining method (e.g., Wright-Giemsa, Papanicolau, rapid Romanowsky staining) is preferable to others, local expertise and practice should be considered when selecting the slide staining technique; (III) smears, cell block or core tissue can be useful for molecular testing (cell block or core tissue are indispensable to assess ALK translocation, while smear may be successfully used to evaluate the status of EGFR when cell block or tissue core are lacking).
The adequacy rate of EBUS-TBNA for molecular analysis depends on many factors: small sample size, tumor necrosis, sampling of nodal micrometastasis, and contamination of the samples with blood or bronchial cells (32). Several studies demonstrated that molecular analysis can be routinely performed on the majority of samples obtained by EBUS-TBNA with successful rate ranging from 89% to 98% (36,37). Other studies have demonstrated the feasibility of performing complete molecular tests, with or without IHC on EBUS-TBNA sample (38) with respect to other minimally (39) or more invasive procedures (31). However, a question remains: how much tissue is needed? It depends on the history of patient disease because after the diagnosis and IHC and EGFR/ALK analyses, it should be necessary to perform other molecular investigation (i.e., resistance molecular alterations) or the material should be used for patient clinical study enrollment.
Nowadays, we are still limited by the tissue availability because a single step assay is frequently used. In the near future next generation comprehensive analyses will allow multiplex assays with higher speed, higher sensitivity, and high throughput diagnostics results using much minor amount of tissue (40). However, some validation, cost-efficiency and standardization issues should be resolved and they are much near than we think (41).
Concluding remarks
EBUS-TBNA has emerged as an innovative technique which has been successfully introduced into our daily clinical practice giving several advantages including minimally invasive approach, safe, cost-effective, real time image guidance, and broad sampling capability. It has also acquired importance considering cases in which surgical procedures are contraindicated or unnecessary. Using EBUS-TBNA we are now able to perform an accurate diagnosis associated to the identification of prognostic and predictive morphological and molecular markers. Considering that about 70% of patients with lung cancer are not candidate to surgery but they may be treated with new target therapies, the introduction of an increasing number of molecular tests will continue to transform our daily practice with the need of even more molecular determination using even low amount of adequate material. The use of EBUS-TBNA could satisfy those requests with safe and cost efficiency.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- VanderLaan PA, Wang HH, Majid A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): an overview and update for the cytopathologist. Cancer Cytopathol 2014;122:561-76. [Crossref] [PubMed]
- Monaco SE. Introduction to EBUS-TBNA. In: Monaco SE, Khalbuss WE, Pantanowitz L. editors. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): a practical approach. Basel: Karger, 2014:1-3.
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Casal RF, Staerkel GA, Ost D, et al. Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest 2012;142:568-73. [Crossref] [PubMed]
- Joseph M, Jones T, Lutterbie Y, et al. Rapid on-site pathologic evaluation does not increase the efficacy of endobronchial ultrasonographic biopsy for mediastinal staging. Ann Thorac Surg 2013;96:403-10. [Crossref] [PubMed]
- da Cunha Santos G, Ko HM, Saieg MA, et al. "The petals and thorns" of ROSE (rapid on-site evaluation). Cancer Cytopathol 2013;121:4-8. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Saegusa F, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for nodal staging in patients with lung cancer. Ann Thorac Surg 2013;95:1695-9. [Crossref] [PubMed]
- Guo H, Liu S, Guo J, et al. Rapid on-site evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of hilar and mediastinal lymphadenopathy in patients with lung cancer. Cancer Lett 2016;371:182-6. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration 2013;85:486-92. [Crossref] [PubMed]
- Sarode V, Chau D, Duey M, et al. Rapid On-site Evaluation (ROSE) of Cytology Smears Performed by Cytotechnologists for Assessment of Adequacy in Deep-seated Lesions: Correlation with Final Interpretation. J Am Soc Cytopathol 2016;5:S60. [Crossref]
- van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth edition. Lyon: IARC Press, 2015.
- Whithaus K, Fukuoka J, Prihoda TJ, et al. Evaluation of napsin A, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med 2012;136:155-62. [Crossref] [PubMed]
- Righi L, Vavalà T, Rapa I, et al. Impact of non-small-cell lung cancer-not otherwise specified immunophenotyping on treatment outcome. J Thorac Oncol 2014;9:1540-6. [Crossref] [PubMed]
- Karlsson A, Brunnström H, Lindquist KE, et al. Mutational and gene fusion analyses of primary large cell and large cell neuroendocrine lung cancer. Oncotarget 2015;6:22028-37. [Crossref] [PubMed]
- Tsao AS, Scagliotti GV, Bunn PA Jr, et al. Scientific Advances in Lung Cancer 2015. J Thorac Oncol 2016;11:613-38. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Remon J, Morán T, Majem M, et al. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: a new era begins. Cancer Treat Rev 2014;40:93-101. [Crossref] [PubMed]
- Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7. [Crossref] [PubMed]
- Vatrano S, Righi L, Vavalá T, et al. Molecular and Histological Changes in Post-Treatment Biopsies of Non-Squamous Non-Small Cell Lung Cancer: A Retrospective Study. Target Oncol 2016;11:157-66. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Jukna A, Montanari G, Mengoli MC, et al. Squamous Cell Carcinoma "Transformation" Concurrent with Secondary T790M Mutation in Resistant EGFR-Mutated Adenocarcinomas. J Thorac Oncol 2016;11:e49-51. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Clinical Lung Cancer Genome Project (CLCGP). Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci Transl Med 2013;5:209ra153. [PubMed]
- Marchetti A, Ardizzoni A, Papotti M, et al. Recommendations for the analysis of ALK gene rearrangements in non-small-cell lung cancer: a consensus of the Italian Association of Medical Oncology and the Italian Society of Pathology and Cytopathology. J Thorac Oncol 2013;8:352-8. [Crossref] [PubMed]
- Champiat S, Ileana E, Giaccone G, et al. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol 2014;9:144-53. [Crossref] [PubMed]
- Remon J, Chaput N, Planchard D. Predictive biomarkers for programmed death-1/programmed death ligand immune checkpoint inhibitors in nonsmall cell lung cancer. Curr Opin Oncol 2016;28:122-9. [Crossref] [PubMed]
- Casadio C, Guarize J, Donghi S, et al. Molecular Testing for Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Suitability of Endobronchial Ultrasound Transbronchial Needle Aspiration. Am J Clin Pathol 2015;144:629-34. [Crossref] [PubMed]
- Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- José RJ, Shaw P, Taylor M, et al. Impact of EBUS-TBNA on modalities for tissue acquisition in patients with lung cancer. QJM 2014;107:201-6. [Crossref] [PubMed]
- Jeyabalan A, Bhatt N, Plummeridge MJ, et al. Adequacy of endobronchial ultrasound-guided transbronchial needle aspiration samples processed as histopathological samples for genetic mutation analysis in lung adenocarcinoma. Mol Clin Oncol 2016;4:119-25. [PubMed]
- Rooper LM, Nikolskaia O, Carter J, et al. A single EBUS-TBNA procedure can support a large panel of immunohistochemical stains, specific diagnostic subtyping, and multiple gene analyses in the majority of non-small cell lung cancer cases. Hum Pathol 2016;51:139-45. [Crossref] [PubMed]
- Schmid-Bindert G, Wang Y, Jiang H, et al. EBUS-TBNA provides highest RNA yield for multiple biomarker testing from routinely obtained small biopsies in non-small cell lung cancer patients - a comparative study of three different minimal invasive sampling methods. PLoS One 2013;8:e77948. [Crossref] [PubMed]
- Salto-Tellez M, Gonzalez de Castro D. Next-generation sequencing: a change of paradigm in molecular diagnostic validation. J Pathol 2014;234:5-10. [Crossref] [PubMed]
- Aziz N, Zhao Q, Bry L, et al. College of American Pathologists' laboratory standards for next-generation sequencing clinical tests. Arch Pathol Lab Med 2015;139:481-93. [Crossref] [PubMed]


