Log odds of positive lymph nodes is a novel prognostic indicator for advanced ESCC after surgical resection
Introduction
Esophageal carcinoma (EC) is a common malignancy worldwide, the main type of EC are classified as adenocarcinoma in the West, whereas most cases in Eastern countries are esophageal squamous cell carcinoma (ESCC), such as China, where it accounts for more than 90% of all EC cases (1,2). This implies that there might be differences in the prognosis and therapeutic strategies for EC patients in Eastern countries.
Lymph node (LN) metastasis is considered as one of the most important factors in determining the prognosis of ESCC patients following surgical resection (3). The pathologic nodal (pN) stage of the tumour-node-metastasis (TNM) 7th classification, is still widely used to predicting clinical prognosis of ESCC (4), However, studies have showed that the number of LN metastasis is significantly influenced by the number of dissected LNs, so if the number of LNs detected is insufficient, the accuracy of the pN staging for prognostic assessment will be impacted and led to “stage migration” (5-8). In addition, the definition of the pN0 classification is no positive LN detected. LN negative patients account for a large proportion in the overall esophageal cancer patients, which means that many patients do not survive from the pN classification.
The log odds of positive lymph nodes (LODDS) have been regarded a novel prognostic indicator in many other cancers, such as pancreatic cancer, gastric cancer, and colorectal cancer, defined as the log of the ratio between the probability of being a positive LN and the probability of being a negative LN when one LN is dissected (9-14). However, in dealing with advanced ESCC patients (pN0 stage especially) after curative surgery, rare attention has been paid to the prognostic value of LODDS. Thus, the aim of this study is to determine the prognostic effect of LODDS system in patients with ESCC, and to explore an appropriate classification based on LODDS.
Methods
Patients
The clinical data of 260 patients with histopathologically confirmed ESCC who underwent curative esophagectomy with standard lymphadenectomy at Tianjin Medical University Cancer Institute and Hospital from January 2005 to December 2009 were retrospectively analyzed. The inclusion criteria were as follow: (I) patients who underwent esophagectomy and lymphadenectomy; (II) R0 resection margins determined by microscopic examination of the surgical specimen; (III) thoracic ESCC confirmed by histopathologic examination; (IV) staging of ESCC as T2-4N0-3M0 according to the TNM 7th classification; (V) no preoperative radiotherapy and/or chemotherapy. The exclusion criteria include: (I) patients who underwent palliative surgery; (II) patients who had distant metastasis.
Surgical procedures and pathological examination
In our institution, the preoperative workup included a history, physical examination, endoscopy of the entire upper gastrointestinal tract, chest computed tomography, and ultrasonography/computed tomography of the neck and abdomen. The choice of surgical procedure of esophagectomy was made by surgeon’s preference, and based on the esophageal cancer treatment guidelines. LN dissections were performed using the standard or extended (en-bloc) technique. The dissected thoracic nodes included the recurrent, hilar, carinal, pulmonary ligament, phrenic, and other paratracheal nodes. The abdominal LNs that were dissected included the paragastric and hepatic artery nodes. Dissected LNs were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin-eosin (HE). The LN number was counted by low power field microscopy and the status was determined by experienced pathologists. Postoperative pN staging was according to the AJCC TNM 7th staging system: pN1, patients with 1–2 metastatic LNs; pN2, patients with 3–6 metastatic LNs; pN3, patients with 7 or more metastatic LNs (4). LODDS were estimated using the calculation: log(pnod+0.5)/(tnod-pnod+0.5) in which pnod is the number of positive LNs and tnod is the total number of LNs retrieved.
Follow-up
After curative surgery, all of the patients were followed up by hospital visit, mail, or telephone call every 3 months for the first year, every 6 months for the second year, and then every year until death or the last follow-up. The deadline of the follow-up was set at December 2014. OS time was determined from the date of surgery to the date of death or the last follow-up, otherwise they were considered “lost to follow-up”. A total of 61 patients survived, 152 patients died, 47 cases lost to follow-up were treated as censored data for the analysis of survival rates.
Statistical analysis
Statistical analyses was performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) software. Survival analysis was performed using the Kaplan-Meier method and validated by the log-rank test. The significant clinicopathologic factors were included into the Cox proportional hazards regression model to determine the independent prognostic factors and compute their HRs and 95% CIs. Receiver-operating-characteristic (ROC) curve and area under the curve (AUC) analysis was used to compare the association of pN and LODDS with 3-year or 5-year overall survival (OS). The cut-point analyses were performed to determine whether there was a cutoff LODDS related to the greatest OS difference by use a step analysis with 0.1 LODDS intervals to predict prognosis, and then a series of log-rank tests were performed, with the highest Chi-squared statistic representing the greatest group difference. For all statistical analyses, P values ≤0.05 were considered significant.
Results
Patient characteristics and OS
A total of 260 patients with advanced ESCC undergoing surgical resection were enrolled. The clinical characteristics of these patients are shown in Table 1. There were 216 (83.1%) male patients and 44 (16.9%) female patients with their median age of 62 years (range, 27 to 92 years). Overall, 44 (16.9%) patients were in stage pT2, 133 (51.2%) patients were in stage pT3 and 83 (31.9%) patients were in stage pT4. and 155 (59.6%) patients had node-negative disease and 105 (40.4%) had nodal metastases. A total of 2,893 positive LNs were dissected, and 715 of these were positive (24.7%). The median LODDS were −1.20 (range, −1.97 to 0.95). All patients were divided into four groups according to the quartiles of LODDS (continuous variable). Thus, there were 74 patients in LODDS1 (range, −1.97 to −1.43), 67 patients in LODDS2 (range, −1.43 to −1.20), 55 patients in LODDS3 (range, −1.20 to −0.69), and 64 patients in LODDS 4 (range, −0.69 to 0.95).
The median follow-up period for the entire cohort was 30 months (range, 3 to 106 months). The cumulative 1-, 3-, and 5-year survival rates for all the patients were 70.0%, 41.9%, and 30.3%, respectively, with the median survival time was 27 months.
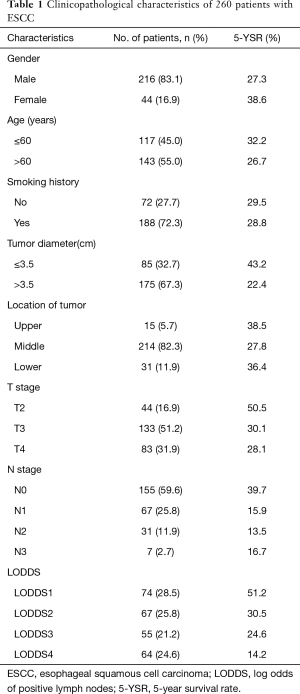
Full table
Prognostic value of LODDS in advanced ESCC
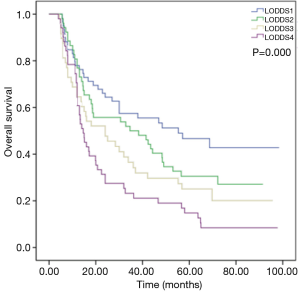
The log-rank test shows LODDS considerably influenced survival. The 5-year OS rates were 51.2%, 30.5%, 24.6%, and 14.2% in LODDS1, LODDS2, LODDS3, and LODDS4, respectively, and the median survival times were 68.7, 34.6, 24.0, and 14.6 months, respectively (Figure 1, P=0.000).
To determine whether LODDS were an independent factor associated with OS in ESCC patients, univariate Kaplan-Meier analysis and multivariate analysis was performed to assess the predictive capability of each variable. The univariate and multivariate analyses with respect to OS also indicated tumor diameter [hazard ratio (HR) =0.681, P=0.038], T stage (HR =1.243, P=0.012) and LODDS (HR =1.309, P=0.003) to be independent and significant prognostic factors in all patients, and T stage (HR =2.010, P=0.019) and LODDS (HR =1.610, P=0.038) in node-negative patients (Table 2).
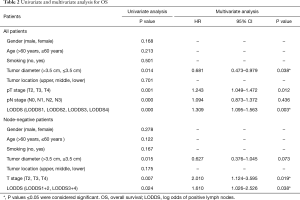
Full table
Subgroup analysis
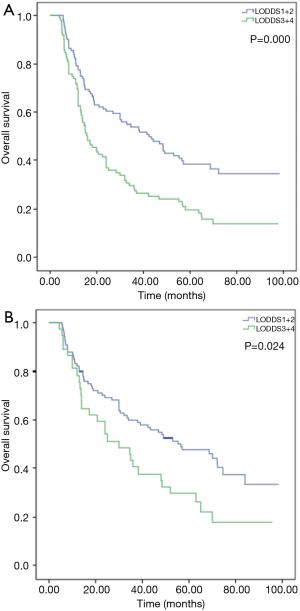
Subgroup analysis showed no significantly difference in survival between LODDS1 and LODDS2 (P=0.096) or between LODDS3 and LODDS4 (P=0.109). To study furthermore, LODDS 1 and 2 (−1.97.0 to −1.20) were combined, and LODDS 3 and 4 (−1.20 to 0.95) were combined. First, of all patients, the 5-year OS rates were 40.7% and 19.1%, respectively, and the median survival times were 42.4 and 15.7 months, respectively (Figure 2A, P=0.000). Then we examined the prognostic effect of LODDS according to different pN stages. The results indicated that higher LODDS were associated with worse OS in patients with pN0 stage (Figure 2B, P=0.024), but the difference was not statistically significant in pN1–3 stage patients (P=0.570).
ROC analyses
The AUC based on ROC curves for 5-year OS or 3-year OS were measured and compared using the method established by DeLong et al. (15). ROC analyses showed that the AUC of LODDS stage (AUC =0.630, P=0.001) was larger than that of pN stage (AUC =0.621, P=0.003) in prediction of 3-year OS, the difference were not statistically significant (Figure 3A, Z=0.164, P=0.565). However, the AUC of LODDS stage (AUC =0.620, P=0.010) was smaller than that of pN stage (AUC =0.631, P=0.005) in prediction of 5-year OS, the differences were also not statistically significant (Figure 3B, Z=0.300, P=0.618).

Cut-off points of LODDS
Lastly, we determined the best cut-off point for the LODDS by using a step analysis with 0.1 LODDS intervals and comparing OS rates for all and node-negative patients. The 5-year OS based on the different cutoff points for LODDS are shown in Table 3. In all patients, the distribution of the cut-points was irregular, so we could not define an optimal cut-off point. However, in node-negative patients, the result shows a cut-off point was −1.2 with statistically significant (Table 3, P=0.024), and the ROC analysis also indicated that a cut-off value of −1.2 for LODDS provided the highest sensitivity and specificity interestingly (Figure 3C).
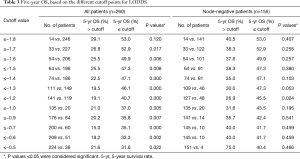
Full table
Discussion
LN metastasis has be deemed to one of the most important factors in predicting the prognosis of ESCC patients following surgical resection (3), and the pN classification based on the number of involved LNs is still widely used. However, studies have showed that the number of LN metastasis is significantly influenced by the number of dissected LNs, so if the number of LNs detected is insufficient, the accuracy of the pN staging for prognostic assessment will be impacted and led to “stage migration” (5-8). Recently, the metastatic LN ratio (MLR) which was defined as the ratio of the metastatic LNs to the total dissected LNs was proposed to evaluate the prognosis of ESCC (16-18). He et al. compared the use of pN stage and the MLR for determination of the prognosis of 353 patients with ESCC who were positive for LN metastasis following radical esophagectomy, and the results showed that a high MLR was independently and significantly associated with poor 5-year OS (19). However, prognostic value of MLR remains controversial (20). In addition, the MLR0 stage is defined as no positive LN involved that is the same as the pN0 stage (21). LN negative patients account for a large proportion in the overall esophageal cancer patients, which means that many patients do not survive from the two stage method.
The LODDS are defined as the log of the ratio between the probability of being a positive LN and the probability of being a negative LN when one LN is dissected, and has been proposed to be recently a superior prognostic indicator (compare to the pN or MLR classification) in other cancer, such as gastric cancer (15,16), pancreatic cancer and colorectal cancer (18-20). In addition, LODDS are not affected by the number of LN negative or positive, therefore, LODDS seem to have more advantages in terms of predicting prognosis of patients without LN metastasis. Recently, the prognostic value of LODDS in ESCC has received increased attention. Wu et al. (22) examined 589 patients who received radical surgery of ESCC, and used six multivariate models comparing the prognostic performance of different LN staging systems in determination of the OS, including all of the five LN classifications (pN, LNR, RLNS, NLNs, and LODDS). Their team found that the LODDS classification was a superior predictor to pN staging system. However, no consensus has been reached about the role of LODDS in the disease because of the limitations in sample size, evaluation criteria, and variety of cut-off values.
Our findings are consistent with above research partly. The results showed that LODDS were an independent association between LODDS and OS after surgical resection for ESCC. Invariably, a high LODDS were significantly associated with poor 5-year OS, which is observed in all patients. Due to the limited number of node negative patients, when divided into four groups, there may be zero packet. To avoid this situation, LODDS1 and 2 (−1.97.0 to −1.20) were combined, and LODDS3 and 4 (−1.20 to 0.95) were combined in subgroup analysis, and the results showed the effect did not change in 155 patients without LN metastasis. Meanwhile, the multivariate analysis showed that pT staging was also an independent risk factor associated with the prognosis of advanced ESCC. Therefore, pT and LODDS staging can be used as a comment predictor of OS of patients. However, further ROC analysis shows the differences of relevance of pN and the LODDS with 5- or 3-year OS were not statistically significant. The difference between our findings and previous studies may be due to differences in the patient selection criteria. For example, Wu’s study excluded patients with T4 stage ESCC, so the relevance of their findings to these patients is unclear.
Although LODDS are important in terms of prognosis, the optimal cut-off points have not yet been established. In our study, two methods were adopted to determine the optimal cut-off value for tumor size. In the first model, 0.1 LODDS were set as the standard interval, and 12 cut-off points were checked one by one. After evaluating the two groups by log-rank test, we identified the distribution of the cut-points was irregular in total patients regretfully, suggesting that there is no definite cut-point after which the survival difference gradually decreased. Thus, we could not define an optimal cut-off point for all patients. Fortunately, we were first to obtain a cut-off point with the highest Chi-squared value from clinical data of node-negative patients. In the second method, as shown by the ROC curve, the cut-off point of −1.2 for LODDS could provide the best compromise between specificity and sensitivity.
However, our study had several potential limitations. It was a retrospective study in nature with a relatively small sample population, leaving some groups small in the statistical analyses. In addition, our data originated from a single institution but with different surgeons and pathologists. Furthermore, a standardized guideline and regimen for postoperative therapy was lacking during the period of this study. Because of this deficiency, we did not evaluate the potential survival benefit possibly related to adjuvant therapy. Further investigations should be performed involving a larger sample size, randomized prospective cohorts, and practical methods from multicenter institutions to determine the optimal cut-off point and confirm these preliminary results in the future.
In conclusion, our findings show that the LODDS system is a novel prognostic indicator for advanced ESCC patients, especially in node-negative cases. And we showed that the histologically determined −1.2 may be the optimal cut-off point for LODDS. It offers some helpful suggestions for pN0 stage patients risk classification, identifying patients at high risk who would be candidates for additional preoperative or postoperative therapy.
Acknowledgements
Funding: This work is supported by a grant from the National Key Clinical Specialist Construction Programs of China (No. 2013-544).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Because it was a retrospective study in nature with a relatively small sample population, the ethic approval is waived. The patients were followed by telephone, and all patients had provided written informed consent for the use of their information in the hospital database.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg 2003;76:S1367-9. [Crossref] [PubMed]
- Takeno S, Yamashita SI, Yamamoto S, et al. Number of metastasis-positive lymph node stations is a simple and reliable prognostic factor following surgery in patients with esophageal cancer. Exp Ther Med 2012;4:1087-91. [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol 2014;21:2857-63. [Crossref] [PubMed]
- Twine CP, Lewis WG, Morgan MA, et al. The assessment of prognosis of surgically resected oesophageal cancer is dependent on the number of lymph nodes examined pathologically. Histopathology 2009;55:46-52. [Crossref] [PubMed]
- Kong SH, Lee HJ, Ahn HS, et al. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Ann Surg 2012;255:50-8. [Crossref] [PubMed]
- Lin CS, Cheng CT, Liu CY, et al. Radical Lymph Node Dissection in Primary Esophagectomy for Esophageal Squamous Cell Carcinoma. Ann Thorac Surg 2015;100:278-86. [Crossref] [PubMed]
- Sun Z, Xu Y. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010;116:2571-80. [Crossref] [PubMed]
- Qiu MZ, Qiu HJ, Wang ZQ, et al. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS One 2012;7:e31736. [Crossref] [PubMed]
- La Torre M, Nigri G, Petrucciani N, et al. Prognostic assessment of different lymph node staging methods for pancreatic cancer with R0 resection: pN staging, lymph node ratio, log odds of positive lymph nodes. Pancreatology 2014;14:289-94. [Crossref] [PubMed]
- Arslan NC, Sokmen S, Canda AE, et al. The prognostic impact of the log odds of positive lymph nodes in colon cancer. Colorectal Dis 2014;16:O386-92. [Crossref] [PubMed]
- Song YX, Gao P, Wang ZN, et al. Which is the most suitable classification for colorectal cancer, log odds, the number or the ratio of positive lymph nodes? PLoS One 2011;6:e28937. [Crossref] [PubMed]
- Wang J, Hassett JM, Dayton MT, et al. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg 2008;12:1790-6. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Wang N, Jia Y, Wang J, et al. Prognostic significance of lymph node ratio in esophageal cancer. Tumour Biol 2015;36:2335-41. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Wu SG, Li FY, Zhou J, et al. Prognostic value of different lymph node staging methods in esophageal squamous cell carcinoma after esophagectomy. Ann Thorac Surg 2015;99:284-90. [Crossref] [PubMed]
- He Z, Wu S, Li Q, et al. Use of the metastatic lymph node ratio to evaluate the prognosis of esophageal cancer patients with node metastasis following radical esophagectomy. PLoS One 2013;8:e73446. [Crossref] [PubMed]
- Tan Z, Ma G, Yang H, et al. Can lymph node ratio replace pn categories in the tumor-node-metastasis classification system for esophageal cancer? J Thorac Oncol 2014;9:1214-21. [Crossref] [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Wu SG, Sun JY, Yang LC, et al. Prognosis of patients with esophageal squamous cell carcinoma after esophagectomy using the log odds of positive lymph nodes. Oncotarget 2015;6:36911-22. [PubMed]


