Tumor invasiveness defined by IASLC/ATS/ERS classification of ground-glass nodules can be predicted by quantitative CT parameters
Introduction
Advances of technology in computed tomography (CT) have significantly increased the detection of early stage lung cancers with subsolid nodules on CT scans (1), which can be further classified as pure ground glass nodules (pGGNs) or part-solid nodules (PSNs) (2).
The International Association for the Study of Lung Cancer, American Thoracic Society and European Respiratory Society (IASLC/ATS/ERS) proposed a new pathology classification of lung adenocarcinoma in 2011, which represents the evolving advances in stratifying lung adenocarcinoma based on tumor morphology (3). A relationship between CT findings and pathology was observed that the ground glass portion of subsolid nodule correlated to the noninvasiveness of lung adenocarcinoma. However, the majority of these researches was based on the Noguchi classification or 2004 WHO classification (4,5). Several studies using 2011IASLC/ATS/ERS classification focused on the differentiation of atypical adenomatous hyperplasia (AAH) from invasive pulmonary adenocarcinoma (IPA) (6), or differentiation of preinvasive lesion (AAH and AIS) from IPA via visual inspection of nodule features and linear measurements (7). Thus, it is still challenging for surgeons to correctly judge the invasiveness of subsolid nodules with objective and reliable parameters, rendering determining optimal procedure and the appropriate timing for surgical intervention.
Development of multidetector CT (MDCT) and computer technology have made quantitative image analysis possible for subsolid nodules. The goal of this study is to investigate the value of employing differentiating CT parameters, including quantitative and qualitative, to differentiate noninvasive pulmonary adenocarcinomas from IPAs by analyzing subsolid nodules retrospectively.
Methods
Patients
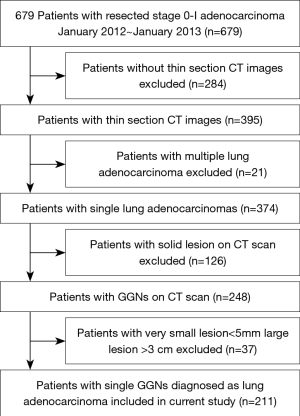
This study was approved by the Ethics Committee of the Shanghai Chest Hospital, and the patients’ written informed consent was waived by the Ethics Committee. From January 2012 to January 2013, a total of 679 resected and pathologically proved stage 0-I lung adenocarcinoma patients were in our center. Thereafter, multiple lung adenocarcinomas; solid lesion on CT scan; lesions <5 mm or >3 cm on CT scan were excluded. Finally, 211 patients with a single lung adenocarcinoma (5 mm < T < 3 cm) whose MDCT scans appeared as subsolid nodules were included (Figure 1). All patients were not preoperatively treated with chemotherapy or radiotherapy, 56 were men (mean age, 55.18±10.06 years; range, 29–80) and 155 were women (mean age, 54.74±9.41 years; range, 30–74 years). Histopathology was based on the 2011IASLC/ATS/ERS classification of lung adenocarcinoma. In this study, adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) were defined as noninvasive adenocarcinoma (8), and IPAs were defined as invasive adenocarcinoma including lepidic predominant, acinar predominant, papillary predominant and other subtypes.
CT examination
Unenhanced helical CT scans were performed with a 64-detector row scanner (Brilliance, Philips, Cleveland, USA).The parameters of routine CT were as follows: detector collimation, 0.625 mm × 64; pitch, 1.08; 120 kV and 250 mA; 5–7 s scan time; SFOV, 400 mm; section thickness and interval, 5.0 and 5.0 mm; filter function C, DFOV, 363 mm; matrix, 512×512. When a lung nodule was found, a HRCT target scan was performed by the same CT scanner with following parameters: collimation, 0.625 mm ×64; pitch, 0.64, 120 kV and 300 mA; 1–3 s scan time; SFOV, 180 mm; section thickness and interval, 1.0 and 0.5 mm, range 5 cm; filter function F,DFOV, 180 mm; matrix, 1,024×1,024. Images were reconstructed by “standard” and “sharp” algorithms for routine CT and targeted HRCT scans respectively. Analysis of CT density was conducted by a computer graphics support system (Tivew software. WinningSoft, Shanghai, China).
Evaluation of CT features
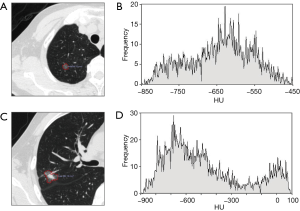
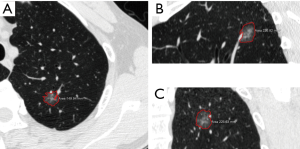
Two chest radiologists (Z.Z.C., and Y.J.D., with 15 and 31 years of experience in chest imaging, respectively), blinded to the histological results, assessed CT images on lung windows (level, −520 HU; width, 1,450 HU). The average values of quantitative parameters including CT size, area and CT value measured by two radiologists were applied for analysis. When discrepancies of morphological parameters of two radiologists occurred, decisions were reached by consensus. All subsolid nodules were classified as pGGN or PSN. pGGN was defined as a shadow completely occupied by a hazy increased attenuation of the lung, with reservation of the bronchial and vascular margins in the lesion with no solid regions. PSN was defined as a heterogeneous attenuation with an internal solid component completely obscuring the underlying lung parenchyma. The normal structures such as vessels and bronchioli within or around the nodule were eliminated manually when performing CT value analyzing. CT findings of each lesion were analyzed as follows: (I) one-dimensional mean CT value (attenuation values of pGGN and PSN, manually indicating the border of the biggest area of interest on axial section with subsequent quantitative analysis by computer program) (Figure 2); (II) maximum CT value (for PSN, the maximum attenuation values within the border of the biggest area of the nodule on axial section); (III) three dimensional (3D) CT value (for PSN, mean CT value of the biggest area of interest of axial, coronal and sagittal section were measured, the average value of the three sections was the 3D CT value) (Figure 3); (IV) location; (V) multiplicity (solitary, multiple); (VI) size (the largest diameter on axial section); (VII) area; (VIII) solid component area (for PSN, the largest solid component area on axial section); (IX) solid proportion (for PSN, dividing the solid component area by the lesion area); (X) margin (spiculated, nonspiculated); (XI) border (lobulated, nonlobulated); (XII) bubble lucency; (XIII) pleural indentation; (XIV) vascular change (dilated, rigid, convergent and tortuous); and (XV) spinous protuberance.
Statistical analysis
pGGN and PSN were separated to perform statistical analysis. Patients’ sex and CT qualitative features between noninvasive and invasive adenocarcinoma were compared by Pearson X2 or Fisher exact test. Patients’ age, pT size and CT quantitative parameters were compared by using unpaired t-test. The optimal cut-off values were calculated by receiver operating characteristic (ROC) curve analysis. Multiple logistic regression analysis was conducted to ascertain which factors differentiate noninvasive adenocarcinoma from IPA, and the parameters with P<0.10 were input as variables. Weighted k statistic was used to test the interobserver agreement for qualitative CT findings, and the degree of agreement was according to Landis and Koch (9). Concordance correlation coefficients (CCCs) were calculated for interobserver agreement for measurements of CT size, CT area, solid component area, 1D mean CT value, 3D mean CT value, 1D maximum CT value. Statistical analysis was performed by using SPSS, version 19.0 (SPSS, Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
Results
Clinical and pathological features were listed in Table 1. There were no significant differences in demographic findings between noninvasive adenocarcinomas and IPAs appearing as pGGN or PSN. The pathological size of lesion was significantly smaller in noninvasive adenocarcinomas compared to IPAs in both pGGO and PSN.
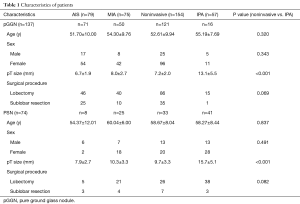
Full table
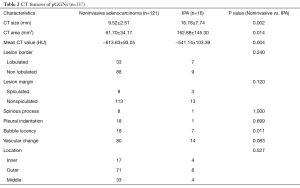
CT features of pGGNs
Noninvasive adenocarcinomas were significantly smaller in CT size (P=0.002) and CT area (P=0.014). One-dimensional mean CT value was significantly lower in noninvasive adenocarcinomas (P=0.004). The presence of bubble lucency was significantly less frequent in noninvasive adenocarcinomas in comparison to IPAs (P=0.011). Other morphologic features were not significantly different between noninvasive adenocarcinomas and IPAs. Tables 2,3 summarized the differentiating CT features between noninvasive adenocarcinomas and IPAs appearing as pGGNs. There was only one patient diagnosed as IPA with pGGN less than 10 mm, whose CT size and CT value is 6.89 mm and −515.85 Hu, respectively.
Full table
Full table
ROC analysis and multiple logistic regression of pGGNs
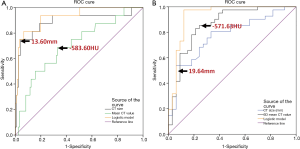
ROC analysis demonstrated that the area under the ROC curve (AUC) for CT size and CT area was 0.890 (95% confidence interval: 0.780, 1.000) and 0.893 (95% confidence interval: 0.796, 0.991), and the optimal cutoff value of CT size and CT area for differentiating noninvasive adenocarcinomas from IPAs was less than 13.60 mm (sensitivity, 75.0%; specificity, 99.6%) and 82.40 mm2 (sensitivity 87.5%, specificity 82.9%). AUC for one-dimensional mean CT value is 0.703 (95% confidence interval: 0.565, 0.840), and the optimal cutoff value for differentiating noninvasive adenocarcinomas from IPAs was less than −583.60 HU (sensitivity, 68.8%; specificity, 66.9%) (Figure 4).
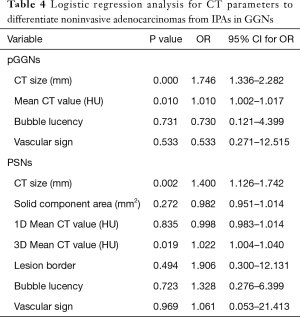
Because CT size was closely correlated to the CT area, the CT area was removed from the multiple regression analysis. CT size, one-dimensional mean CT value, lesion margin, bubble lucency and vascular sign were input as independent variables for multivariate analysis. Multivariate regression revealed that CT size (P=0.000; 95% confidence interval: 1.336, 2.282; odds ratio, 1.746) and one-dimensional mean CT value (P=0.010; 95% confidence interval:1.002, 1.017; odds ratio, 1.010) were the predictive factors of noninvasive adenocarcinomas compared to IPAs (Table 4). The P values of lesion margin, bubble lucency and vascular change were 0.996, 0.731, 0.533, respectively.
Full table
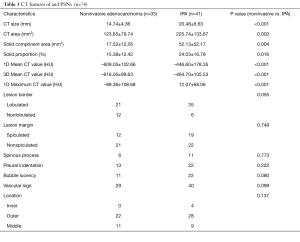
CT features of PSNs
Among the CT features, noninvasive adenocarcinomas were significantly smaller in CT size: (P<0.001), and CT area (P=0.002). Noninvasive adenocarcinomas had significant smaller solid component area (P=0.004) and solid proportion (P=0.016) in comparison to IPAs. One-dimensional mean CT value (P<0.001) and maximum CT value (P<0.001), and 3D mean CT value (P<0.001) were significantly lower in noninvasive adenocarcinomas. There was no significant difference with regard to morphologic features between noninvasive adenocarcinomas and IPAs. Tables 2,3 concluded the differentiating CT features between noninvasive adenocarcinomas and IPAs appearing as PSNs.
ROC analysis and multiple logistic regression of PSNs
ROC analysis showed that the AUC for CT size and CT area was 0.773 (95%confidence interval: 0.665, 0.880) and 0.789 (95%confidence interval: 0.684, 0.898), and the optimal cutoff value of CT size and CT area for differentiating noninvasive adenocarcinomas from IPAs was less than 19.64 mm (sensitivity, 53.7%; specificity, 93.9%) and 169.93 mm2 (sensitivity 63.4%, specificity 87.9%), respectively. AUC for solid component area and solid proportion was0.778 (95%confidence interval: 0.674, 0.880) and 0.652 (95% confidence interval: 0.526, 0.778) respectively. The optimal cutoff value of solid component area and solid proportion for differentiating noninvasive adenocarcinomas from IPAs was less than 19.045 mm2 (sensitivity, 78.0%; specificity, 66.7%) and 21.365% (sensitivity 53.7%, specificity 81.8%), respectively. AUC for mean CT value and maximum CT value of axial section, and 3D CT value is 0.832 (95% confidence interval: 0.737, 0.928), 0.776 (95% confidence interval: 0.665, 0.888), and 0.860 (95% confidence interval: 0.772, 0.949), respectively. The optimal cutoff value of one-dimensional mean CT value and maximum CT value of axial section, and 3D mean CT value for differentiating noninvasive adenocarcinomas from IPAs was less than –546.13 HU (sensitivity, 75.6%; specificity, 81.8%), −14.00 HU (sensitivity 56.2%,specificity 75.8%), and −571.63 HU (sensitivity, 85.4%; specificity, 75.8%), respectively (Figure 4).
Because the CT size was closely correlated to the CT area, and the same relation as solid component area and solid proportion, the CT area and solid component area were removed from the multiple regression analysis. CT size, solid proportion, one-dimensional mean and maximum CT value, 3D mean CT value, lesion border, bubble lucency and vascular change were input as independent variables (Table 4). CT size (P=0.002; 95% confidence interval: 1.126, 1.742; odds ratio, 1.400) and 3D mean CT value (P=0.019; 95% confidence interval: 1.004, 1.040; odds ratio, 1.022) were the significant differentiating factors of noninvasive adenocarcinomas compared to IPAs.
Interobserver agreement analysis for CT findings of subsolid nodules
The k value of qualitative CT morphologic features of subsolid nodules varied between 0.475~0.898. The CCCs for quantitative CT parameters were very high, between 0.892~0.998. Table 5 summarized the results of interobserver agreement analysis for CT findings of the subsolid nodules.
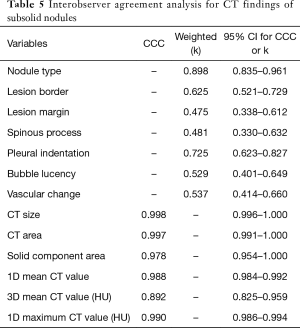
Full table
Discussion
The imaging description of 2011 IASLC/ATS/ERS entities were insufficient in describing lesions in nature. It was reported that AIS and MIA have a 100% disease-free survival (DFS), which is much lower in IPAs (10,11). Thus, we included AISs and MIAs into the noninvasive adenocarcinomas. The present study studied qualitative and quantitative parameters to determine the CT features to predict the invasiveness of lung adenocarcinoma. Based on our results, quantitative parameters may enable surgeons to predict tumor invasiveness of subsolid nodules to aid in clinical decision-makings pertaining to the timing of surgical intervention and extent of resection.
A recent study reported that tumor invasiveness defined by IASLC/ATS/ERS classification correlated with the 5-year survival data of p-stage IA lung adenocarcinoma (8). In our study, there was significant difference between noninvasive adenocarcinomas and IPAs in CT size and area, one-dimensional mean CT value and bubble lucency in pGGNs. Lim et al. found that there was no significant difference among AIS, MIA and IPA with regard to CT size and morphologic features in pGGNs (12). Lee et al. reported that CT size and lesion border were significantly different between preinvasive lesions and IPAs in pGGNs (7). The presence of bubble lucency indicated the nodules that grew over an interval of 2 years (13). As for PSNs, we found that CT size and area, solid component area, solid proportion, one-dimensional mean CT and maximum CT value, and 3D mean CT value were significantly different between noninvasive adenocarcinomas and IPAs. In Lee’s study, there were significant differences in lesion size, solid proportion and morphologic features such as lesion margin, border and pleural retraction between preinvasive lesions and IPAs (7). None of the morphologic features was significant with regard to tumor invasiveness for PSNs in present study.
Many radiological studies reported a strong relationship between the degree of GGO and pathology, however, so far there has been no consensus with regard to the best measurement metric to predict the tumor invasiveness and patient survival. Suzuki et al. had classified small adenocarcinoma into six subtypes according to GGO extent, however, having only the degree of GGO is insufficient to evaluate all subsolid nodules (14). Tumor disappearance rate (TDR) was also used to examine the ratio of solid component by linear measurement of area on mediastinal window and lung window settings (15). In fact, many subsolid nodules were non-discernible on mediastinal window view settings, which made it difficult to use TDR to evaluate such lesions. Morphological parameters appeared to be inconsistent predictors for the pathology of lung adenocarcinoma (16). Therefore, objective and quantitative metrics are needed to analyze subsolid nodules.
Increased CT value is an important predictive factor for tumor invasiveness (1). However, using CT value in the management of GGNs was debated for the influence of different CT protocols such as section thickness and the use of contrast-medium (17). In our study, all the CT images were unenhanced by 1 mm thin section CT (TSCT), which complied with the Fleischner Society guideline’s requirement (2). For pGGNs, we chose one-dimensional mean CT value to differentiate the invasiveness due to its homogeneous characteristic (18). The only patient diagnosed as IPA with pGGN under 10 mm, invasiveness could be identified via mean CT value, which was −515.85 HU (cutoff value is −583.60 HU). Two-dimensional and 3D quantitative analysis had been used to evaluate the CT attenuation or CT number based histogram to differentiate among AAH, BAC and IPA (19). Ikeda et al. reported that the 3D mean CT value was the more useful for differentiating BAC from IPA (20). For PSNs, we found that using the CT value as a differentiator was better than using CT size with regard to the accuracy of ROC analysis, especially the 3D mean CT value seemed to be with the highest accuracy (sensitivity, 85.4%; specificity, 75.8%).
When and how subsolid lesions should be resected still remained controversial. The size of the lesion alone has been believed not to be an accurate predictive factor of tumor invasiveness (21). We found that mean CT value coupled with CT size were optimal quantitative parameters to predict tumor invasiveness, and the cutoff values in this study were close to previous reports. Suzuki et al. suggested that 15 mm was the resection criterion for pGGNs (14). Kitami et al. reported the cutoff value of CT size and one-dimensional mean CT value were 10 mm and −600 HU based on Noguchi classification (18). It is reasonable that our results were slightly higher than Kitami’s data because we had included MIA into noninvasive adenocarcinoma. The interim guidelines of pulmonary GGNs by Godoy recommended that 10mm or larger is the criterion for surgical resection for pGGNs (22). We suggest that for pGGNs, if the CT size >13.60 mm, and mean CT value >−583.60 HU, surgery (lobectomy) is indicated, because the lesion was highly diagnosed as IPA. If the CT size of pGGNs is between 10 and 13.60 mm, one-dimensional mean CT value would be a recommendation for treatment strategy. Because there was some overlap between noninvasive and invasive adenocarcinomas with regard to CT features, even for pGGN less than 10 mm, mean CT value will be still critical for the treatment decision. Multivariate logistic analysis also revealed that CT size and 3D mean CT value were predictive of noninvasive adenocarcinomas from IPAs for PSNs, and the cutoff was 19.64 mm and −571.63 HU, respectively. We suggested that the combination of CT size and 3D mean CT number would also be helpful for the surgical procedure options for PSNs (20). Interestingly, although the difference of cutoff value of CT size between pGGNs and PSNs was not small (13.60 vs. 19.64 mm), the cutoff value of mean CT value between pGGNs and PSNs was close (−583.60 vs. −571.63 HU). Because the diagnosis of GGN is subjective sometime, it is noteworthy that the mean CT value reflecting the true nature of the GGN lesion is critical for the management tactics.
There were several limitations in our study. First, we have not included the benign lesions. However, the operation criteria for GGNs based our results have made us to resect lung adenocarcinoma in 95% patients with GGNs (data not show). Second, 3D mean CT value in this study was “semiautomated”, in which we manually indicated the area of nodule while the computer software did the quantitative analysis subsequently for each dimension. “Automated” technology that facilitates the 3D segmentation of subsolid nodule might play an important role in the future. Last, to eliminate all vessels, bronchioli and air bronchograms perfectly when performing GGN analyzing seemed not possible, and this might partly cause the interobserver variation.
In conclusion, tumor invasiveness of GGNs as defined by IASLC/ATS/ERS classification can be predicted by quantitative CT parameters. In pGGNs, CT size and one-dimensional mean CT value are determinants for tumor invasiveness. In PSNs, invasiveness can be predicted by CT size and 3D mean CT value of the nodule.
Acknowledgements
We thank Prof. Qiang Shen from Department of Clinical Cancer Prevention, Division of Cancer Prevention and Population Sciences, The University of Texas MD Anderson Cancer Center for the language revision of this paper.
Funding: This work was supported by the Program of Science and Technology Commission of Shanghai Municipality 15ZR143-8200 and 16DZ2345900.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of the Shanghai Chest Hospital, and the patients’ written informed consent was waived by the Ethics Committee.
References
- Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology 2001;220:803-9. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Travis WD, Garg K, Franklin WA, et al. Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma. J Clin Oncol 2005;23:3279-87. [Crossref] [PubMed]
- Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics 2007;27:391-408. [Crossref] [PubMed]
- Lee SM, Park CM, Goo JM, et al. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology 2013;268:265-73. [Crossref] [PubMed]
- Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. [Crossref] [PubMed]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules >/= 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Takahashi S, Tanaka N, Okimoto T, et al. Long term follow-up for small pure ground-glass nodules: implications of determining an optimum follow-up period and high-resolution CT findings to predict the growth of nodules. Jpn J Radiol 2012;30:206-17. [Crossref] [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. [Crossref] [PubMed]
- Honda T, Kondo T, Murakami S, et al. Radiographic and pathological analysis of small lung adenocarcinoma using the new IASLC classification. Clin Radiol 2013;68:e21-6. [Crossref] [PubMed]
- Lee SM, Park CM, Goo JM, et al. Transient part-solid nodules detected at screening thin-section CT for lung cancer: comparison with persistent part-solid nodules. Radiology 2010;255:242-51. [Crossref] [PubMed]
- Nakamura S, Kawata H, Nanbu R, et al. Investigation of Region of Interest (ROI) for measurement of slice thickness in Computed Tomography (CT). Nihon Hoshasen Gijutsu Gakkai Zasshi 2010;66:217-24. [Crossref] [PubMed]
- Kitami A, Kamio Y, Hayashi S, et al. One-dimensional mean computed tomography value evaluation of ground-glass opacity on high-resolution images. Gen Thorac Cardiovasc Surg 2012;60:425-30. [Crossref] [PubMed]
- Nomori H, Ohtsuka T, Naruke T, et al. Differentiating between atypical adenomatous hyperplasia and bronchioloalveolar carcinoma using the computed tomography number histogram. Ann Thorac Surg 2003;76:867-71. [Crossref] [PubMed]
- Ikeda K, Awai K, Mori T, et al. Differential diagnosis of ground-glass opacity nodules: CT number analysis by three-dimensional computerized quantification. Chest 2007;132:984-90. [Crossref] [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [Crossref] [PubMed]
- Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 2009;253:606-22. [Crossref] [PubMed]