Transthoracic echocardiography is a safe alternative for assessment and guidance of transcatheter closure of secundum atrial septal defect in children
Introduction
Transcatheter device closure of secundum atrial septal defects (ASD) is the first-line method of repair when the anatomy is suitable given the lower morbidity rate in comparison to surgery (1,2). Over- or under-sized device increased the risk of complication (3). A careful assessment of ASD size, morphology and rims is the key-step for device selection. Trans-esophageal echocardiography (TEE) or intracardiac echocardiography (ICE) are widely used to guide the procedure (4,5). However, TEE use may lead to oropharyngeal and oesophageal traumas (6-8), and ICE is expensive and require an additional large venous access (9). Given a good echocardiographic window in children, transthoracic echocardiographic (TTE) guidance has been proposed (10-13). However this approach is not universally accepted (14,15) and most publications report either small or old series, always on selected patients (10-13). We aimed in this study to compare efficacy and safety of 2D-TTE versus 2D-TEE to guide percutaneous ASD closure in a large, unselected, consecutive pediatric population.
Methods
Study design
We conducted a retrospective single-center comparative study at the Marie-Lannelongue Hospital in Paris, France. All patients <15-year-old who underwent attempt of transcatheter isolated, secundum ASD closure with the Amplatzer Septal Occluder (ASO) between 1998 and 2014 were included. Data were collected with special attention to echocardiographic data, implantation characteristics, early and long-term outcomes. The study database was reported to the French data protection authority (CNIL No. 1154338, April 27, 2006). Our study complies with the Declaration of Helsinki. Our institutional review board approved the study (No. CCML 2015-4), and all patients or legal guardians gave their informed consent to study inclusion.
Pre-operative echocardiographic assessment
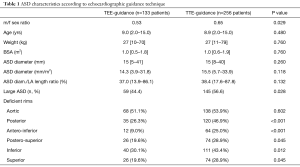
ASD rims and size were assessed by preoperative TTE in all patients. Right ventricular size and function were assessed according to American Society of Echocardiography guidelines (16). Right ventricular systolic pressure was estimated using the Bernoulli equation in patients with tricuspid regurgitation. The ASD diameter was measured in apical and sub-costal four-chamber views, parasternal-short axis view and subcostal bi-caval view. The ASD diameters were measured at the end-systolic frame of the cardiac cycle (17) and the largest ASD diameter on any view was recorded as ASD size. ASO/LA (left atrial) length ratio was defined as the ratio between the diameter of the left atrial (LA) disc of the device and the LA length. LA length was the distance from the anterior mitral valve leaflet to the posterior LA wall on the subcostal view. When not measured, LA length was estimated as 0.597+0.404·log (Body Surface Area) (18). Very large ASD was defined as an echocardiographic diameter ≥15 mm/m2 in children (19). Device closure was attempted to treat very large ASDs in 204 (52.4%) children.
We labeled ASD rims as proposed by Amin and universally accepted, as aortic (or superoanterior), anteroinferior (or atrioventricular valve), inferoposterior (or inferior venacaval), posterior, posterosuperior (or superior venacaval), and superior (20,21). Special attention was given to anteroinferior and posterosuperior rims on the four-chambers view, aortic and posterior rims on the short-axis left parasternal view, and inferoposterior and superior rims on the subcostal view. Rims less than 5 mm in length were considered deficient (20). Anteroinferior, posterosuperior, aortic, posterior, inferoposterior and superior rims were deficient in 76 (19.5%), 100 (25.7%), 206 (52.9%), 155 (39.8%), 151 (38.8%) and 100 (25.7%) patients respectively.
Procedure characteristics and per-operative echocardiographic guidance
The procedure was performed as previously described (19,22-24). From 1998 to 2005, ASD closure was done in all children (n=133, 34.2%) under general anesthesia with oro-tracheal intubation and 2D-TEE guidance. From 2005 to 2014, 2D-TTE guidance was used in all children (n=256, 65.8%), regardless of age or ASD characteristics. TTE-guided procedures were performed under deep sedation in 213 (54.7%) spontaneously breathing children younger than 12 years and under local anesthesia in the other 43 (11.1%) patients.
Right-sided cardiac catheterization was performed to evaluate hemodynamics and exclude additional congenital heart defects. Ten (2.6%) patients had pulmonary arterial hypertension (defined as mean pulmonary arterial pressure >25 mmHg). Oxygen saturations were analyzed in 197 (50.6%) children, consistently showing substantial left-to-right inter-atrial shunting with a median Qp/Qs ratio of 2.5 (1.0–4.0). In patients with pulmonary hypertension, criteria for ASD closure were pulmonary vascular resistance <15 WU·m2, persistent left-to-right inter-atrial shunt (Qp/Qs ratio >1.5), and symptoms onset within the past 6 months (25).
Device choice was based on balloon sizing. Balloon sizing was used in all patients regardless of whether TTE or TEE was utilized. Balloon sizing was performed in all patients using the “pull-through” technique (26). A 27 mm or 33 mm Medi-Tech Equalizer balloon (Boston Scientific, Natick, MA, USA) was fully inflated in the left atrium with diluted contrast and then gently pulled back against the septum. With progressive balloon deflation, a slight deformity of the balloon was seen on fluoroscopy, just prior to its popping through the septum, giving the balloon-stretched diameter of the ASD (27). Median balloon-stretched diameter was 21 mm (range, 8–40 mm) namely 20.9 mm/m2 (range, 8.1–48.2 mm/m2). Balloon-stretched diameter/LA length ratio was 53.5% (range, 22.4–98.1%).
Amplatzer Septal Occlusion device was utilized for all the patients. An appropriate sized device was deployed with fluoroscopic guidance, using a standard, previously described placement protocol (19). ASO/LA length ratio was <0.8 in 142 (36.5%) patients, 0.8 to 0.9 in 98 (25.2%), 0.9 to 1 in 67 (17.2%), and >1 in 82 (21.1%).
TTE was used only after device deployement and before its release. TTE was used to evaluate the position of each disk and potential impingement of the device on adjacent cardiac structures. The relation of the device to the atrioventricular valves and the aorta was assessed using the parasternal short-axis, the apical 4-chambers and the subcostal views. Color Doppler assessment of residual shunting, systemic and pulmonary venous return including coronary sinus, and atrioventricular valve function were also assessed, as applicable in these views. Gentle pushing and pulling of the delivery cable was performed to ensure that the device was in a secure position. The device was released only if the echocardiogram demonstrated a correct position without evidence of significant residual shunting, atrioventricular valve malfunction, or venous obstruction.
Procedural success was defined as presence of all three following criteria: successful ASO delivery without peri-procedural complications; well-positioned ASO as assessed by TTE after 6 and 48 hours, with no ASO migration; and hospital discharge on post-procedure day-1. Cardiac erosion, pericardial effusion, air embolus, ASO-related valvular regurgitation, thromboembolism, pulmonary edema, stroke, atrioventricular block, ventricular arrhythmias, and hemolysis were considered major complications. At hospital discharge, patients were prescribed oral antiplatelet therapy for 6 months.
Follow-up
ECG was checked at day-1. A physical examination, 12-lead ECG and TTE were performed 6 and 24 hours post-procedure. A physical examination, 12-lead ECG and TTE were done 1 week, 3 months, and 1 year post-procedure. The referring cardiologists provided subsequent follow-up. Long-term outcomes were assessed by telephone interviews of all patients and all referring cardiologists to obtain information on cardiac status, data at the last visit, and any delayed complications.
Statistical analysis
Analysis were performed using StatEL software (www.adscience.eu, adScience, Paris, France). Categorical variables were described as numbers and percentages. Continuous variables were expressed as median (min-max). Data regarding TEE-guided and TTE-guided procedures were compared using the nonparametric Mann-Whitney test. Categorical variables were compared using chi2 test and Fischer exact test. Successful and failed procedures were also compared according to rim deficiencies using the chi2 test and Fischer exact test. A p-value <0.05 was considered statistically significant.
Results
Demographic and ASD characteristics of the 389 pediatric procedures are detailed in Table 1. Hemodynamic and device characteristics are detailed in Table 2. Linear regression analysis demonstrated that the mean balloon-stretched diameter was greater of 5.59±1.38 mm/m2 in the TTE-guided group than that of the TEE-guided group (P<0.001).
Full table

Full table
Immediate post-procedural outcome
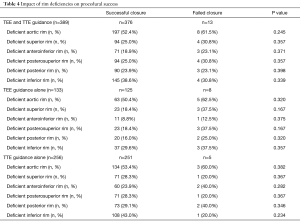
The procedure was successful in 376 (96.7%; 95% CI: 94.4–98.2%) patients (Table 3). A trivial residual inter-atrial shunt was observed in 8 children (2.1%, 95% CI: 0.9–4.1%). In the 204 children with very large ASD, the procedure was successful in 196 (96.1%; 95% CI: 92.4-98.3%) cases, 143 (70.1%) of them being TTE-guided. Results of this specific subgroup have been reported in details elsewhere (18). Rim deficiencies were not associated with procedural failure (Table 4).

Full table
Full table
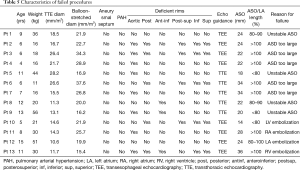
The procedure failed in 13 (3.3%; 95% CI: 1.8–5.6%) of the 389 children (Table 5). The ASO was deployed but not delivered in 9 (5 TTE-guided, 4 TEE-guided) then considered unstable or too large to be closed and withdrawn, without incident. In these cases the ASO was retrieved before being delivered, to avoid cardiac deformation or device-related atrioventricular valve regurgitation. ASO migration occurred in the remaining 4 patients (1.0% 95% CI: 0.3–1.6%; 1 in the right atrium, 1 in the right ventricle, 1 in the left atrium and 1 in the left ventricle), of whom 3 were managed by transcatheter ASO retrieval and surgical ASD closure and 1 by same-stage surgical ASO retrieval and ASD closure. All the 4 patients whose device embolized had had TEE-guided procedure (P=0.013). No other major or minor complications occurred during the procedure or within the first 48 post-procedural hours in these patients. A 10-year-old, 21-kg boy who had a successful TEE-guided ASD closure with a 24 mm-ASO associated to an Ebstein disease, underwent surgical device retrieval and ASD closure via a sternotomy at the post-procedural day 11 because of severe device-related hemolysis. This complication was favored by a lateralized tricuspid regurgitation jet directed against the device. Overall, early major adverse events were observed in 5 patients (1.3% 95% CI: 0.4-3.0%), all in the TEE group (P=0.004).
Full table
Procedural outcome according to the echocardiographic guidance technique
Children whose procedure was TTE-guided had significantly more posterior, anteroinferior, posterosuperior, inferoposterior and superior deficient rims (Table 1). In the TTE-guided group, very large ASDs were more frequent and both balloon-stretched diameter and ASO/LA length ratio were greater than in TEE-guided procedures (Tables 1,2).
The procedural success rate tended to be higher in the TTE-guidance group (98.0%, 95% CI: 95.5–99.4% vs. 94.0%, 95% CI: 88.5–97.4%, P=0.069). ASO migrations occurred only in case of TEE-guided ASD closure (3.0%; 95% CI: 0.8–7.5% vs. 0.0%, 95% CI: 0.0–1.4%, P=0.013) (Table 5). The failure rate due to excessively large or unstable ASO was not significantly different between the TEE and TTE groups (P=0.498) (Table 3). In both groups, rim deficiencies were not associated with procedural failure (Table 4).
Long-term outcome
After a median follow-up of 7 years (range, 1–16 years), no patient was lost to follow-up. All patients were alive and asymptomatic and no one had been rehospitalized for device-related complication. None experienced late complications; more specifically, no cases of cardiac perforation or ASO migration were recorded. No regurgitation through the aortic, mitral, or other valves developed in any patient. There were no instances of ASO-related endocarditis, thromboembolism, conduction disorders, or ventricular arrhythmias. After ASD closure, 67 women had 75 uneventful pregnancies; more specifically, no intra- or postpartum thromboembolic events occurred. All patients had normal left ventricular systolic function at the last visit. One (0.3%, 95% CI: 0–1.4%) 12-year- old patient developed atrial fibrillation 5 years after the procedure, controlled effectively by pharmacological treatment.
Discussion
Echo guidance of ASD closure
TEE was required in the pivotal trial of the ASO device and continues to be the most widely used technology for ASD assessment, device selection, and guidance during implantation (2,28,29). However the rational to consider TTE guidance as an alternative is driven by some rare reports of TEE-related esophageal perforation in adults and children (6-8), as well as the fact that TTE-guidance may allow to shorten both procedure time and fluoroscopy time (30) and to avoid general anesthesia and orotracheal intubation in spontaneously breathing children (30,31). Additionally, one can present the argument that in TTE-guided procedures, the interventionist may be able to complete the whole process himself without the need for an additional echographist and without additional irradiation (10,30). We report here a routine experience on 2D-TTE guided ASD closure in 256 unselected children compared to 133 2D-TEE guided procedures. Our main finding is that in experienced hands, 2D-TTE associated with balloon-sizing is a valuable alternative to 2D-TEE for ASD closure guidance. Our results are in accordance to other groups (30,31).
TTE-guidance is not universally accepted and published data are limited to either small series or selected patients (10-12). However one monocenter prospective randomized study suggested non-inferiority of 2D-TTE compared to 2D-TEE in 40 selected children (11).
In 2D-TTE guided procedures, devices were positioned under fluoroscopy only, whereas in 2D-TEE guided procedures, real-time TEE images of the ASD and surrounding structures such as the aorta root were available for the interventionist, thus theoretically facilitating the positioning. Our results underline the pivotal role of the method of choice of the device, which is closely related to procedural success and complication rate. 2D-TEE and 2D-TTE images underestimate the ASD maximal size compared to balloon-sizing (5,32-34). In our experience, a balloon calibration was performed in all cases minimizing the impact of the echo technique on the device choice.
Obviating the need for the transesophageal echocardiography in TTE-guided procedures seems not to have impaired the decision making process. This makes TTE-guided procedure a highly interesting alternative strategy, especially when percutaneous ASD closure is performed in developing countries, where expensive techniques such as ICE or TEE are not easily affordable (35,36).
Our successful ASD device implant rate of 96.7% was similar to procedural success rates of 95.7% in the IMPACT Registry, 96% in the MAGIC report, 95% in the C3PO report, and 95.7% in the Amplatzer Septal Occluder FDA study (2,29,37,38). Major adverse events were observed in 1.3% in our study. This rate compares with 1.2% in IMPACT patients, 1.1% in MAGIC patients, 4.7% in C3PO patients and 1.6% in the FDA study (2,29,37,38). As previously described, device embolization was successfully managed percutaneously in most cases (34). Moreover we did not observe late adverse events. Although rare, late complications like conduction abnormality, endocarditis, thrombo-embolism or aortic valve regurgitation have been occasionally described (38-40). Device related aortic erosion remains a dreaded complication occurring in 0.5% in the literature (40). In our study, TEE and TTE guided procedures seems to compare favorably with regards to both early and long-term outcomes.
Limitations
We focused on severe early and delayed complications. Complete data on supraventricular arrhythmias were not collected. Given the large number of patients coming from all parts of the country, detailed follow-up of cardiac rhythm with regular 24-hour ECG Holter monitoring would not have been feasible in all patients. Our study describes two different groups of patients with different ASD characteristics and from two different eras (namely 1998-2005 for TEE-guidance and 2005-2014 for TTE-guidance). As experience has evolved with time, complications observed in the TEE group may have been part of the learning curve of the ASD closure technique.
Conclusions
2D-TTE is safe and efficient to guide percutaneous ASD closure in unselected children. This modified technique allows a shortened and simplified procedure performed in spontaneously breathing children. Our results strongly support the fact that 2D-TTE can be considered as an efficient alternative to 2D-TEE for assessment and guidance of pediatric ASD closure in experienced centers.
Acknowledgements
Dr. Baruteau is supported by a research grant from the European Society of Cardiology. The authors gratefully acknowledge Dr. Jérôme Petit, Dr. Dominique Piot, Dr. Virginie Lambert and Dr. Jean Losay (from Marie-Lannelongue Hospital in Paris, France) for their implication in patients’ management.
Footnote
Conflicts of Interest: Dr. Fraisse is a proctor and consultant for St Jude Medical. Dr. Baruteau received a research grant from St Jude Medical. Other coauthors have no conflict of interest to disclose.
Ethical Statement: Our institutional review board approved the study (No. CCML 2015-4), and all patients or legal guardians gave their informed consent to study inclusion.
References
- Butera G, Biondi-Zoccai G, Sangiorgi G, et al. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention 2011;7:377-85. [Crossref] [PubMed]
- Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, et al. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 2002;39:1836-44. [Crossref] [PubMed]
- Amin Z, Hijazi ZM, Bass JL, et al. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv 2004;63:496-502. [Crossref] [PubMed]
- Seo JS, Song JM, Kim YH, et al. Effect of atrial septal defect shape evaluated using three-dimensional transesophageal echocardiography on size measurements for percutaneous closure. J Am Soc Echocardiogr 2012;25:1031-40. [Crossref] [PubMed]
- Hascoet S, Hadeed K, Marchal P, et al. The relation between atrial septal defect shape, diameter, and area using three-dimensional transoesophageal echocardiography and balloon sizing during percutaneous closure in children. Eur Heart J Cardiovasc Imaging 2015;16:747-55. [Crossref] [PubMed]
- Kumar G, Provenzano S, Alphonso N, et al. Esophageal perforation associated with fontan operation: a complication of transesophageal echocardiography. World J Pediatr Congenit Heart Surg 2013;4:293-5. [Crossref] [PubMed]
- Ayres NA, Miller-Hance W, Fyfe DA, et al. Pediatric Council of the American Society of the Echocardiography. Indications and guidelines for performance of transesophageal echocardiography in the patient with pediatric acquired or congenital heart disease: report from the task force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2005;18:91-8. [Crossref] [PubMed]
- Sainathan S, Andaz S. A systematic review of transesophageal echocardiography-induced esophageal perforation. Echocardiography 2013;30:977-83. [Crossref] [PubMed]
- Assaidi A, Sumian M, Mauri L, et al. Transcatheter closure of complex atrial septal defects is efficient under intracardiac echocardiographic guidance. Arch Cardiovasc Dis 2014;107:646-53. [Crossref] [PubMed]
- Erdem A, Saritas T, Zeybek C, et al. Transthoracic echocardiographic guidance during transcatheter closure of atrial septal defects in children and adults. Int J Cardiovasc Imaging 2013;29:53-61. [Crossref] [PubMed]
- Bartakian S, El-Said HG, Printz B, et al. Prospective randomized trial of transthoracic echocardiography versus transesophageal echocardiography for assessment and guidance of transcatheter closure of atrial septal defects in children using the amplatzer septal occluder. JACC Cardiovasc Interv 2013;6:974-80. [Crossref] [PubMed]
- Kardon RE, Sokoloski MC, Levi DS, et al. Transthoracic echocardiographic guidance of transcatheter atrial septal defect closure. Am J Cardiol 2004;94:256-60. [Crossref] [PubMed]
- Watanabe N, Taniguchi M, Akagi T, et al. Usefulness of the right parasternal approach to evaluate the morphology of atrial septal defect for transcatheter closure using two-dimensional and three-dimensional transthoracic echocardiography. J Am Soc Echocardiogr 2012;25:376-82. [Crossref] [PubMed]
- Johri AM, Witzke C, Solis J, et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr 2011;24:431-7. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714-833. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Franke A, Kuhl HP, Rulands D, et al. Quantitative analysis of the morphology of secundum-type atrial septal defects and their dynamic change using transesophageal three-dimensional echocardiography. Circulation 1997;96:II-323-7. [PubMed]
- Hanséus K, Bjorkhem G, Lundstrom NR, et al. Cross-sectional echocardiographic measurement of right atrial and right ventricular size in children with atrial septal defect before and after surgery. Pediatr Cardiol 1988;9:231-6. [Crossref] [PubMed]
- Baruteau AE, Petit J, Lambert V, et al. Transcatheter closure of large atrial septal defects: feasibility and safety in a large adult and pediatric population. Circ Cardiovasc Interv 2014;7:837-43. [Crossref] [PubMed]
- Amin Z. Transcatheter closure of secundum atrial septal defects. Catheter Cardiovasc Interv 2006;68:778-87. [Crossref] [PubMed]
- Vaidyanathan B, Simpson JM, Kumar RK. Transesophageal echocardiography for device closure of atrial septal defects: case selection, planning, and procedural guidance. JACC Cardiovasc Imaging 2009;2:1238-42. [Crossref] [PubMed]
- Losay J, Petit J, Lambert V, et al. Percutaneous closure with Amplatzer device is a safe and efficient alternative to surgery in adults with large atrial septal defects. Am Heart J 2001;142:544-8. [Crossref] [PubMed]
- Harper RW, Mottram PM, McGaw DJ. Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc Interv 2002;57:508-24. [Crossref] [PubMed]
- Hanslik A, Pospisil U, Salzer-Muhar U, et al. Predictors of spontaneous closure of isolated secundum atrial septal defect in children: a longitudinal study. Pediatrics 2006;118:1560-5. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Mullins CE. Transcatheter Atrial Septal Defect Occlusion. Cardiac Catheterization in Congenital Heart disease: Pediatric and Adult. 2006, pp 732-739.
- Masura J, Gavora P, Formanek A, et al. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 1997;42:388-93. [Crossref] [PubMed]
- Tobis J, Shenoda M. Percutaneous treatment of patent foramen ovale and atrial septal defects. J Am Coll Cardiol 2012;60:1722-32. [Crossref] [PubMed]
- Everett AD, Jennings J, Sibinga E, et al. Community use of the amplatzer atrial septal defect occluder: results of the multicenter MAGIC atrial septal defect study. Pediatr Cardiol 2009;30:240-7. [Crossref] [PubMed]
- Erdem A, Sarıtas T, Zeybek C, et al. Transthoracic echocardiographic guidance during transcatheter closure of atrial septal defects in children and adults. Int J Cardiovasc Imaging. 2013;29:53-61. [Crossref] [PubMed]
- Li GS, Kong GM, Wang YL, et al. Safety and efficacy of transcatheter closure of atrial septal defects guided by transthoracic echocardiography: a prospective study from two Chinese Medical Centers. Ultrasound Med Biol. 2009;35:58-64. [Crossref] [PubMed]
- Rao PS, Langhough R. Relationship of echocardiographic, shunt flow, and angiographic size to the stretched diameter of the atrial septal defect. Am Heart J 1991;122:505-8. [Crossref] [PubMed]
- Godart F, Rey C, Francart C, et al. Two-dimensional echocardiographic and color Doppler measurements of atrial septal defect, and comparison with the balloon-stretched diameter. Am J Cardiol 1993;72:1095-7. [Crossref] [PubMed]
- El-Said HG, Bezold LI, Grifka RG, et al. Sizing of atrial septal defects to predict successful closure with transcatheter cardioSEAL device. Tex Heart Inst J 2001;28:177-82. [PubMed]
- Zanjani KS, Zeinaloo A, Malekan-Rad E, et al. Transcatheter atrial septal defect closure under transthorasic echocardiography in children. Iran J Pediatr. 2011;21:473-8. [PubMed]
- Praz F, Wahl A, Schmutz M, et al. Safety, feasibility, and long-term results of percutaneous closure of atrial septal defects using the Amplatzer septal occluder without periprocedural echocardiography. J Invasive Cardiol. 2015;27:157-62. [PubMed]
- Moore JW, Vincent RN, Beekman RH 3rd, et al. NCDR IMPACT Steering Committee. Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the national cardiovascular data registry. J Am Coll Cardiol 2014;64:2439-51. [Crossref] [PubMed]
- El-Said H, Hegde S, Foerster S, et al. Device therapy for atrial septal defects in a multicenter cohort: Acute outcomes and adverse events. Catheter Cardiovasc Interv 2015;85:227-33. [Crossref] [PubMed]
- Suda K, Raboisson MJ, Piette E, et al. Reversible atrioventricular block associated with closure of atrial septal defects using the Amplatzer device. J Am Coll Cardiol 2004;43:1677-82. [Crossref] [PubMed]
- Schoen SP, Boscheri A, Lange SA, et al. Incidence of aortic valve regurgitation and outcome after percutaneous closure of atrial septal defects and patent foramen ovale. Heart 2008;94:844-7. [Crossref] [PubMed]


