Intraoperative care for aortic surgery using circulatory arrest
Introduction
Ascending aortic and/or aortic arch surgery is a complex procedure that usually requires a circulatory arrest (CA) to achieve a surgical field that is free of cannulas and clamps. During this period there is an important risk of ischemia of all organs, especially of the central nervous system. The International Aortic Arch Surgery Study Group has published a system of organic dysfunction grading to avoid duplication of overlapping results and to limit fluctuating classifications of negative outcomes among institutions by providing standardized definitions (1).
The first measure used to reduce the risk of ischemia was systemic hypothermia. Subsequently, selective cerebral perfusion methods were used to increase the CA safety time.
Initially hypothermia was produced by external cooling and was first applied by Lewis and Taufic in 1953 for the correction of a CIA in a 5-year-old girl, with surface cooling and with a 5-minute stop.
The development of cardiopulmonary bypass (CPB) has allowed to manage blood temperature and obtain hypothermia more quickly and accurately to reduce perfusion flows until we reach temporary CA. In 1975 Griepp et al. (2) published the first series of aortic arch operations using profound hypothermic CA and in 1986 Guilmet et al. in Europe and Kazui in Japan, successfully introduced selective cerebral perfusion, which allowed the use of warmer temperatures and shorter CPB times.
Other adjuvant measures are possible pharmacological protection, acid-base management and glycemic control.
The main use of CA is in cardiac surgery, especially in surgery on ascending aorta and aortic arch and in congenital heart disease in children. However, there are also other indications (Table 1).
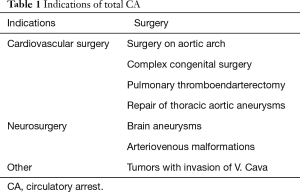
Full table
The safe duration of CA is controversial and will depend on the hypothermia achieved and the concomitant use of cerebral perfusion.
The main pathophysiological aspects involved in cerebral ischemia-reperfusion are the consumption of adenosine triphosphate (ATP), the excitotoxic action of glutamate, the alterations of ionic homeostasis and the formation of oxygen free radicals. Measures that disrupt this cascade of events will theoretically have neuroprotective potential. However, a second pathway of neuronal death by apoptosis has been found, where mitochondria plays a major role and a third pathway due to necroptosis or programmed necrosis that does not depend on caspases. All this would explain that some measures that seem effective may have no long-term impact, because what they do is delay neuronal death.
Hypothermia
Hypothermia acts by decreasing intracellular enzyme activity and cerebral metabolism rate for O2 (CMRO2), thereby improving the balance between supply and demand for oxygen. For each degree of temperature decrease the CMRO2 decreases an average of 6–7%, so at 25 °C the CMRO2 decreases to 37% and 15 °C to 15% of the basal. Cerebral blood flow (CBF) is also reduced in a linear fashion as opposed to the decrease in the CMRO2 that accelerates below 20 °C (3).
In addition, hypothermia could also have protective effects by other mechanisms, such as decreased release of excitatory neurotransmitters such as glutamate or increased release of inhibitory neurotransmitters such as gamma-aminobutyric acid, in addition to suppressing intracellular calcium intake and decrease the production of oxygen free radicals (4) attenuating the ischemia-reperfusion syndrome and an overregulation of protective genes may be possible (5).
The arterial pathway of CPB, through which hypothermia can be induced in aortic arch surgery, can be established through different arteries, but the most used are the femoral artery and especially the right axillary artery.
This artery provides several benefits such as the possibility of using it for cerebral perfusion anterograde, eliminating the risk of retrograde embolization from the descending aorta, reduces the risk of retrograde dissection, directs the flow to true light by decompressing false light, there is less chance of malposition and hypoperfusion, and it is also an artery that is usually free from atherosclerotic plaques (6).
It is recommended to cannulate the axillary artery through a prosthetic graft previously anastomosed to the artery because it prevents damage to the arterial wall especially in the case of small caliber arteries (7,8).
In any case, there is no clear evidence of the superiority of the axillary artery to the femoral artery, and the choice will depend on the clinical circumstances of the patient and the surgeon’s preferences (9,10).
The type of hypothermia for aortic surgery has been defined in a consensus document (9) as described in Table 2 and serves as a frame of reference.
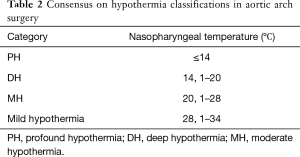
Full table
The degree of hypothermia and the technique used has been varying over time. CA was initially made with profound hypothermia (PH) or deep hypothermia (DH) as the only protection. Table 3 shows the theoretical safety intervals calculated with a ratio of metabolic rates at 2 temperatures 10 °C apart (Q10) of 2.3 (11).
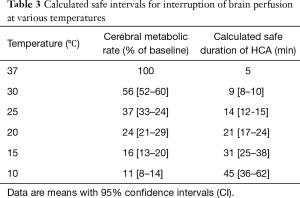
Full table
However, even at 14 °C there are between 14% and 22% of patients that do not reach the electrical silence (12). One study found that in order to achieve cerebral electrical inactivity in >95% of patients it was needed to lower the temperature to 12.7 °C (13).
Subsequently, retrograde cerebral perfusion (RCP) methods were developed through the superior vena cava, to increase the safety time of DH.
More recently, anterograde selective cerebral perfusion methods were started using supra-aortic trunks that allowed the use of moderate hypothermia (MH).
PH (<14 °C): the use of PH did not provide significant advantages, since the decrease in metabolism is small in relation to DH (14) and in addition to all the problems associated with DH, it should be added that it greatly lengthens the CPB time, causes a loss of brain self-regulation and promotes immunosuppression. For these reasons and for the marginal benefit obtained the PH is rarely used today (15).
DH (14.1–20 °C): most patients achieve electrical silence with this temperature range (12).
Using only this technique, without selective cerebral perfusion support, acceptable results were obtained (16), provided that the CA did not last more than 20 or 30 minutes, because after 40 minutes of CA the ACV increased significantly (17), even transient neurological dysfunctions have been reported below 30 minutes (18).
DH also has a number of drawbacks such as high CPB required for cooling and warming of the blood, cerebral microvascular endothelial dysfunction, edema formation, coagulopathy and impaired function of many organs including kidneys, brain, vascular smooth muscle, intestinal mucosa, alveolar epithelium, liver or pancreas (19).
The use of HD as the only protective measure was associated with the occurrence of permanent neurological lesions in 3% to 12% of the patients, renal dysfunction in 5% to 14%, pulmonary insufficiency in 5% to 39% and heart failure in 7% to 34%.
MH (20.1–28 °C): since the use of selective cerebral perfusion methods, more moderate systemic temperatures were started, reducing the CPB time used for cooling and rewarming, and decreasing complications associated with DH previously explained.
Mild hypothermia (28.1–34 °C): some groups have used even warmer temperatures (30±2 °C) with good results in both mortality, neurological deficits and organ failure (20,21).
DH vs. moderate
Due to the disadvantages associated with DH, the use of this technique has been decreasing and CA is currently chosen at higher temperatures associated with specific brain protection strategies because it is the organ most sensitive to ischemia.
In studies comparing DH with MH with selective cerebral perfusion for elective aortic surgery, the latter technique was proven to be safe, with better morbidity and mortality. Thus in a meta-analysis by Tian et al. (22) no differences were found in mortality and temporal neurological dysfunctions, but if an increase of strokes in DH. Halkos et al. (23) and Tsai et al. (24) in each study found a significant decrease in mortality with MH. Immer et al. (25) found that there was an improvement in the quality of life when using cerebral perfusion and warmer temperatures. For his part Vallabhajosyula et al. (26), Milewski et al. (27) and Leshnower et al. (28) did not find significant differences in mortality or neurological alterations between the two techniques, although the first author describes a significant reduction of transfusions with MH and a significantly lower cardiopulmonary bypass (CBP) time.
Another important aspect when using more moderate temperatures is the possibility of insufficient protection of the lower part of the body during CA, especially of the spinal cord and abdominal visceras.
In 2007 Kamiya published a greater tendency towards the appearance of paraplegia with MH in the subgroup of patients with CA over 60 minutes (29). Similar data had already been found in experimental animal studies where it was found that at 28 °C there were up to 60% paraplegia when the PCT was greater than 90 minutes (30).
Table 4 shows the safe times of interruption of the medullary vascularization at different temperatures and calculated according to a Q10 of 2.2 that is almost identical to the cerebral one (11), in fact the DH was used as a method of spinal protection in thoracoabdominal aneurysm surgery (31).

Full table
In recent studies, no significant differences were found in the incidence of renal failure or biomarkers of visceral dysfunction between the two cooling techniques as long as the PCT does not exceed 60 minutes (32,33).
External local cooling
The external cooling of the head is performed generally by surrounding the head with icepacks and is recommended in most protocols. It is an inexpensive and easy to apply method and its theoretical advantage is that it would prevent the transmission of heat by conduction or radiation from hottest objects or tissues. It could also decrease the increase of temperature during the CA because despite the electrical silence can persist a certain degree of metabolic activity and would also lower the temperature gradient between the brain and the operating room environment by preventing brain warming. This situation is important when homogenous tissue cooling has not been achieved before CA, as probably it occurs in some patients (34).
However, there are authors (35) who consider this measure to be of little use, because the skull is a poor conductor of thermal energy and when HM is used with CA the gradient between cerebral and environmental temperature is lower and therefore this measure has less sense. It may also interfere with the placement and operation of sensors used in neurological monitoring.
Management of acid-base balance during hypothermia
Hypothermia increases the solubility of CO2 in plasma and therefore decreases its partial pressure, although the overall content is the same.
If the pH is determined, when pCO2 is corrected to the actual temperature during hypothermia, it is called pH-stat, whereas the non-correction according to temperature is called α-stat.
The α-stat management has a number of advantages such as maintaining cerebral autoregulation and cellular enzyme activity (36) as well as the coupling between CBF and cerebral metabolic oxygen consumption (CMRO2) and is usually the strategy used during the CPB.
The pH-stat implies hypercapnia, with the consequent increase of CBF which can increase the emboligena load and provoke edema.
However, this cerebral vasodilatation can favor the homogeneity of the cerebral cooling, therefore some authors (36,37) recommend using the pH-stat during the cooling period and α-stat in the rest of the CPB including the rewarming. However, there is no strong evidence to recommend a strategy over another.
Temperature management
Following the recommendations of The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of Extracorporeal Technology (38) cooling should be performed gradually by maintaining a temperature gradient between the arterial outlet and the oxygenator venous inlet <10 °C to avoid the generation of gaseous embolism. Also during the rewarming, a gradient of T <10 °C must be maintained until the out-flow temperature reaches 30 °C where it should be lowered to <4 °C.
The oxygenator outlet temperature should not exceed 37 °C to prevent cerebral hyperthermia.
Some authors recommend a cold reperfusion prior to rewarming for at least 10 minutes, especially when DH was used for less than 40 minutes because it appears to reduce neurological events (39).
Selective cerebral perfusion
It is mandatory for MH and optional on DH.
RCP
It was developed in the 80’s and involves reversing the circulatory flow, perfusing oxygenated blood to the brain through the superior vena cava and providing flows between 300 and 500 mL/min with pressures of 25 to 35 mmHg. RCP allows a deep and homogeneous cooling of the brain as well as washing solid particles, air bubbles and metabolites, thereby decreasing acidosis in the ischemic brain (36). Associated with DH has allowed to reduce mortality, stroke (40,41) and delirium significantly with respect to the use of isolated DH (42-44).
The main disadvantage is the risk of cerebral edema due to flow or high perfusion pressures
Antegrade cerebral perfusion (ACP)
ACP consists of delivering oxygenated blood to the brain via the arterial route. It can be unilateral through the right axillary artery, subclavian or innominate or bilateral artery with direct cannulation of the supraaortic trunks.
A flow rate of 6 to 10 mL/kg/min and a perfusion pressure of between 40 and 60 mmHg in the right radial artery or 60 to 70 mmHg in the carotid artery is usually used.
At these pressures and at temperatures above 25 °C, brain self-regulation is usually maintained.
In a meta-analysis where the DH alone was compared with the DH associated with APC, a mortality reduction was aimed at the latter technique, in order to have longer CPB. On the other hand, there were no significant differences in neurological alterations (45).
Retrograde versus ACP
ACP appears to be superior to RCP because it is more physiological with homogeneous distribution of CBF and allows to maintain MH instead of HD. The RCP satisfies only 10% to 20% of the normal perfusion due to the existence of venous shunts, although it may be sufficient to satisfy the demands in case of DH (46). In fact, the beneficial effect of RCP is probably due to maintenance of cerebral hypothermia rather than to metabolic support (47).
The main drawback of ACP is the possibility of cerebral embolisms, by arterial manipulation
Currently ACP is the most commonly used technique for aortic surgery in most hospitals (23,48,49), for its good results (50), and it can increase the safety time to more than 80 minutes (51). It is also described in a propensity matched analysis a reduction of neurological complications and a tendency to lower mortality at 30 days with ACP (52).
However in a meta-analysis of 7,023 patients comparing both types of perfusions, there are no significant differences in permanent neurological dysfunction, stroke or mortality, and the ACP only is superior in the reduction of temporal neurological dysfunction (53).
Based on all these findings, some authors have suggested that RCP can be reserved for cases in which the supra-aortic trunks can not be manipulated by large embolic risk (36).
Unilateral vs. bilateral ACP
The controversy derives from the fact that unilateral ACP may be insufficient in case of carotid stenosis, previous stroke or anatomical abnormalities in the Willis polygon. The bilateral ACP is somewhat more technically difficult and requires more manipulation of the supra-aortic trunks with which the embolic risk is greater.
Studies comparing both perfusions have mixed results. In some publications, a lesser but not significant incidence of stroke with unilateral perfusion has been described (54). However, in a meta-analysis of 5,100 patients (55), no significant differences were found, and in another 6,788 patients (56) the longer CA they were associated with an increase in mortality when the perfusion was unilateral.
The use of one type or another of anterograde perfusion probably depends on the characteristics of each patient, the protocols of each hospital and the existence of asymmetries in regional cerebral oxygen saturation (SrcO2), or electroencephalography (EEG) at start. However, if a CA greater than 40 or 50 minutes is predicted, bilateral perfusion is recommended (57).
Methods of pharmacological cerebral protection
Many drugs have been used for their potential protective capacity such as halogen anesthetics, anti-inflammatories or antioxidants. Frequently the results of experimental studies in the laboratory are promising, however, in prospective, randomized, and controlled studies (36) there is very little evidence of the benefits of its clinical application in humans
Most inhalation anesthetics have neuroprotective properties, by reducing the excitotoxicity as demonstrated by laboratory studies with Isoflurane and Sevoflurane; but at the clinical level this benefit was not demonstrated, especially in the long term (58).
In vitro Xenon reduced the cortical lesion induced by N-Methyl-D-Aspartate receptors (NMDA) or oxygen deprivation in mice (59).
Barbiturates were considered to be the standard protective drug, and they appear to reduce apoptosis in the laboratory, however, there are serious doubts about their true efficacy (60-62), since although they may provide modest neuroprotection, they do not appear superior to others anesthetics and are potentially less effective when associated with hypothermia. However, according to a survey conducted in Europe in 450 centers, it was found that in 60% of the cases are still used (63).
Propofol appears to be neuroprotective in focal and global ischemia models, perhaps because of its antioxidant and anti-inflammatory properties, but as with other anesthetics, clinical efficacy has yet to be demonstrated (64).
Etomidate does not seem to provide any benefit, it can even worsen ischemic injury.
NMDA inhibitors such as ketamine appear to have some protective effect on focal ischemia although published results on its effect in patients after cardiac surgery are contradictory (47,65).
Lidocaine has been used in continuous infusion, but its use is not currently recommended, and may even increase cognitive dysfunction in diabetics (66).
Corticosteroids have been frequently used because of their demonstrated anti-inflammatory effect, however this does not mean that they have a neuroprotective effect, in fact no beneficial effects have been found in ischemic or hemorrhagic stroke or in cardiac surgery (67,68).
Calcium antagonists have also shown no benefit in these procedures.
Magnesium acts by inhibiting glutamate at the NMDA receptor level, reducing intracellular calcium concentrations. A possible benefit was demonstrated in cardiac surgery (69,70) and ischemic stroke (71), although there is also some study with contrary results (72).
Mannitol appears to have a certain antiapoptotic effect in addition to the osmotic and free radical scavenging effect.
The beneficial effects of mannitol, barbiturates and steroids in type A aneurysm surgery in 2,137 patients were examined in the GERAADA database (60) and no neuroprotective effects of any drug could be ascertained; only mannitol was associated with a decrease in mortality after surgery although it may have been due to its effects on other organs.
In a recent review (73) where a large number of studies were analyzed, only statins and magnesium sulfate reduce the incidence of neurological deficits and no pharmacological treatment reduced mortality.
Hyperglycemia
It can worsen neurological lesions by different mechanisms such as increased lactic acidosis tissue, increasing the tissue availability of excitatory amino acids, favoring inflammation and oxidative stress and injuring the cerebral microcirculation.
In addition, it must be taken into account that during CPB it is usual to suffer hyperglycemia even in non-diabetic patients. The Society of Thoracic Surgeons (74) published guidelines for the perioperative control of glycaemia in cardiac surgery, recommending glycemic levels <180 mg/dL, with insulin I.V. in intermittent bolus or better in continuous perfusion, monitoring blood glucose every 30 to 50 minutes. However, intensive treatment with insulin to maintain normoglycemia was shown to increase episodes of hypoglycemia and mortality (75), so it seems reasonable to maintain levels between 140 and 180 mg/dL.
Coagulation
In CA with hypothermia there are multiple coagulation alterations, as those associated with the CPB itself as a decrease in the production of thrombin, consumption of coagulation factors, decrease in the number and malfunction of platelets, fibrinogen deficiency, hyperfibrinolysis and residual effects of heparin, we must add those caused by hypothermia that also causes platelet dysfunction and prolongs prothrombin time and activated partial thromboplastin time. Even in aortic dissections, consumption of coagulation factors in the preoperative period has been reported (76).
For these reasons, all these procedures consume a large amount of blood products for hemostasis.
It is often necessary to replenish clotting factors that can be done with fresh frozen plasma (FFP), prothrombin factor concentrate (PFC) or recombinant factor VII.
The use of FFP is associated with risk of fluid overload and infections, whereas PFC and FVII are associated with a higher risk of thrombosis. In a retrospective study it was shown that with the use of PFC there was less blood loss although it did not result in less transfusion or lower mortality at 30 days and also described a higher incidence of acute renal failure (77), unlike another study where no significant differences were found between the two strategies in the incidence of renal failure that required methods of extrarenal clearance (78).
The use of fibrinogen may be necessary to achieve levels >200 mg/dL which are considered acceptable. The contribution of fibrinogen with FFP is small, so a very large volume of transfusion would be required. The most reasonable alternatives are the use of cryoprecipitates or fibrinogen concentrates.
When the fibrinogen concentrates are used in CA with DH as first-line therapy produce both an increase in fibrinogen levels (79) and a reduction in transfusions (80).
Platelet transfusion is also usually required because platelet dysfunction and hemodilution thrombocytopenia occur during CPB, there is downregulation of IIB/IIIA receptors and mechanical damage, and hypothermia has deleterious effects on platelet function that do not revert immediately after rewarming.
Platelets interact with fibrinogen and transfusion of fibrinogen may be necessary before platelets.
Antifibrinolytic drugs such as lysine analogues have been shown to reduce bleeding and transfusions (81,82) and are present in most protocols; in any case the doses used vary widely according to the centers.
Renal failure and aortic thrombosis have been reported with the use of ε-aminocaproic acid (83).
Monitoring
Usual monitoring will be used following the standards of the American Society of Anesthesiologists, including a pulmonary artery catheter in high risk patients and transesophageal echography (TEE). In addition, in these procedures, neurological and temperature monitoring is important.
It is convenient to monitor blood pressure in both radial arteries, especially if ACP is used through the right axillary artery, which is the most common, since in this case the left radial artery can be used during CPB and right radial artery during ACP (84).
The TEE is very useful for assessing cardiac function, aortic morphology, blood volume, the existence of intracardiac air and surgical repair, in case of aortic dissection the TEE will help us to identify the presence of a dissection flap, the extent of dissection, can allow us to differentiate true from false light and the presence of thrombus in false light. It also allows us to diagnose accompanying injuries such as aortic insufficiency, pericardial effusion and coronary dissection
The ascending aorta can be seen in mid-esophageal planes, short and long axes, and the aortic arch in the upper esophageal plane in short and long axis. However, the superior third of the ascending aorta and proximal part of the arch can not be accurately visualized due to the interposition of the left bronchus. Likewise, TEE is not a good technique for seeing supra-aortic trunks, although there are some alternative projections that improve vision (84).
Neurological monitoring
It should be multimodal and may include brain function monitors such as the EEG or Bispectral Index (BIS), somatosensory evoked potentials (SEPs) and oxygenation-flow monitors and brain metabolism such as transcranial Doppler (TCD), oxygen saturation in the jugular venous sinus (SjO2) and near-infrared spectroscopy (NIRS).
Currently NIRS and BIS are the most used, because they are non-invasive, unlike SjO2, are less complex and require less apparatus than the EEG and SEPs and less subjective than the TCD.
EEG was the main neurological monitoring used in aortic arch surgery with DH to assess electrical activity as a marker of the metabolic suppression produced by hypothermia (85), so that the degree of cooling is marked by electrical silence.
It was verified that the cerebral electrical activity during the cooling follows predictable patterns, although with wide margins (12). Thus, with nasopharyngeal temperatures between 21.5 and 34.2 °C (mean 29.6±3 °C) many patients develop periodic unilateral or bilateral discharge and wave amplitude. With greater cooling there is a gradual decrease in continuity until the appearance of a burst suppression pattern between 15.7 and 33 °C (mean 24.4±4 °C). Finally, with increasing cooling, there is a progression towards complete electrical inactivity between 12.5 and 27.2 °C (mean 17.8±4 °C).
During warming there is a progressive normalization of the EEG but at somewhat different temperatures than during cooling (86).
When using MH with selective ACP the role of the EEG is less established. In these cases CA is usually between 20 and 28 °C and at these temperatures the vast majority of patients have some electrical brain activity. In these cases the EEG can be useful to assess asymmetries or a sudden drop in electrical activity that does not improve with cerebral perfusion and may indicate the performance of other measures such as bilateral ACP or resort to greater cooling (85).
The SEPs have also been used during hypothermic CA. Cortical, subcortical, and peripheral responses may be used during cooling because their suppression should occur prior to CA. The first to disappear is cortical responses, followed by subcortical and peripheral responses (85).
SjO2 can be measured invasively with a sensor inserted into the jugular vein. SjO2 increases as CMRO2 decreases. Maximum metabolic suppression is achieved with saturations above 95%.
TCD is a cheap, non-invasive technique that allows detecting changes in real time, especially embolisms and CBF descents usually during ACP (86). Its main drawbacks are that it requires training and experience, it is dependent operator, it is not easily reproducible and it can be difficult to get a suitable signal.
BIS will serve as a monitor of anesthetic depth and is also useful for controlling brain activity during cooling and rewarming.
With hypothermia the value of BIS decreases, often in a biphasic manner (87) and the suppression rate increases. The range of values varies widely among patients, but usually with temperatures <18 °C the BIS is 0.
Its usefulness in case of hypothermia would be to identify traces of suppression or electrical silence.
The bilateral BIS adds new variables and one of them is the matrix of spectral density where the frequencies and potential of brain waves are represented with a color chart over time and allows the detection of asymmetries between both hemispheres.
NIRS was developed in the 70’s and allows the determination of SrcO2 based on the different absorption properties of light in the near-infrared spectrum of saturated and unsaturated hemoglobin.
Studies done with this monitoring, especially in animals and children, show that SrcO2 increases with the onset of cooling reaching a maximum in most cases after 15 minutes. With the onset of CA there is a decrease in values until circulation is restored and this desaturation correlates with neurohistological damage.
In adults there is less experience, but studies done so far suggest utility (88,89), especially when using selective cerebral perfusion. There are algorithms to increase perfusion flow in case of cerebral desaturation (37) and if it is not enough, bilateral infusion should be used if it was not previously established (90,91). This is why monitoring is used more and more often, although there are some doubts about whether it allows assessing deep brain oxygenation, in fact there seems to be a poor correlation with jugular venous saturation. There are also no data on which threshold or duration of cerebral hypoxia determined by NIRS can be tolerated and compared to the EEG seems to be less sensitive and specific (85).
Temperature monitoring
Several sites have been used for temperature measurement like the tympanum, nasopharynx, esophagus, bladder, rectum and brain. Brain temperature would be the most useful, although difficult.
In a study where hypothermia was used in surgery of cerebral aneurysms (92), it was possible to compare the temperature in the cerebral cortex with other parts of the organism and it was verified that the greatest disparity was with the perfusion temperature and the smallest difference was with the distal esophagus followed by pulmonary artery and nasopharynx. The rectum and bladder were considerably hotter during cooling and colder during warm-up. However, it should be noted that these measurements were performed in closed chest surgeries. In aortic surgery there is loss of heat through the thorax, so in these cases, the temperature closest to the brain is the measurement in the nasopharynx that is also irrigated by branches of the external carotid and therefore comes blood during the ACP.
The thermistor insertion should be through the nares to the level of the midpoint of the zygoma, to a depth of 7 to 10 cm in an adult.
The nasopharynx is a good monitoring site during brain cooling and perfusion, although during warm-up it may underestimate brain temperatura (47), for this reason, it is advisable to control the temperature in two different places and the urinary bladder is often used to measure body temperature although its changes are slower than in the nasopharynx (9).
Coagulation monitoring
Due to the usual coagulation alterations in these procedures, it is interesting to have a point of care that guides our transfusion therapy.
The rotational thromboelastometry (ROTEM) is a point of care assay that examines the viscoelastic properties of whole blood by dynamically measuring clot firmness during its formation and subsequent fibrinilysis.
The development of algorithms based on these measurements has resulted in reduced bleeding, transfusion of blood products and hospital costs (93).
It seems interesting that the determination of fibrinogen assay of ROTEM (FITBEM A10) during CPB has a good correlation with post-CPB fibrinogen levels (68,94) and this would guide the treatment of early hypofibrinogenemia, which could have repercussions on the reduction of allogeneic transfusions (80).
Future
The use of PCT has decreased because nowadays much aortic arch pathology can be performed with hybrid procedures involving either open or endovascular surgery or completely endovascular procedures that do not require CPB or CA.
The hybrid approach consists of a debranching of supra-aortic trunks with a prosthetic tube from native ascending aorta (type I) or from an aortic graft (type II) and at the same surgical act or in a second time the placement of a stent distal to the outlet of the prosthetic tube and covering the complete arch (95).
Endovascular treatment consists of the placement of a thoracic endoprosthesis with anchorage in the ascending aorta and the insertion of chimneys into supraaortic trunks (96).
Conclusions
The CA is a complex procedure, which requires protective techniques against ischemia. The current trend is using MH with ACP through the right axillary artery. Magnesium, statins, or mannitol may be useful, although more studies would be necessary to recommend its routinary use, because evidence of protection in clinical practice is weak.
It is also important to establish protocols, with the rest of the surgical team and the personal of intensive care, for the monitoring and management of the hemodynamic, respiratory parameters, acid-base balance, glycemia, coagulation and temperatura.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yan TD, Tian DH, LeMaire SA, et al. Standardizing clinical end points in aortic arch surgery: a consensus statement from the International Aortic Arch Surgery Study Group. Circulation 2014;129:1610-6. [Crossref] [PubMed]
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [PubMed]
- Kimura T, Muraoka R, Chiba Y, et al. Effect of intermittent deep hypothermic circulatory arrest on brain metabolism. J Thorac Cardiovasc Surg 1994;108:658-63. [PubMed]
- Dorotta I, Kimball-Jones P, Applegate R. Deep hypothermia and circulatory arrest in adults. Semin Cardiothorac Vasc Anesth 2007;11:66-76. [Crossref] [PubMed]
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009;37:S186-202. [Crossref] [PubMed]
- Moizumi Y, Motoyoshi N, Sakuma K, et al. Axillary artery cannulation improves operative results for acute type a aortic dissection. Ann Thorac Surg 2005;80:77-83. [Crossref] [PubMed]
- Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004;78:1274-84. [Crossref] [PubMed]
- Sinclair MC, Singer RL, Manley NJ, et al. Cannulation of the axillary artery for cardiopulmonary bypass: safeguards and pitfalls. Ann Thorac Surg 2003;75:931-4. [Crossref] [PubMed]
- Yan TD, Bannon PG, Bavaria J, et al. Consensus on hypothermia in aortic arch surgery. Ann Cardiothorac Surg 2013;2:163-8. [PubMed]
- Gulbins H, Pritisanac A, Ennker J. Axillary versus femoral cannulation for aortic surgery: enough evidence for a general recommendation? Ann Thorac Surg 2007;83:1219-24. [Crossref] [PubMed]
- Griepp RB, Di Luozzo G. Hypothermia for aortic surgery. J Thorac Cardiovasc Surg 2013;145:S56-8. [Crossref] [PubMed]
- Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 2001;71:14-21. [Crossref] [PubMed]
- James ML, Andersen ND, Swaminathan M, et al. Predictors of electrocerebral inactivity with deep hypothermia. J Thorac Cardiovasc Surg 2014;147:1002-7. [Crossref] [PubMed]
- Mezrow CK, Midulla PS, Sadeghi AM, et al. Evaluation of cerebral metabolism and quantitative electroencephalography after hypothermic circulatory arrest and low-flow cardiopulmonary bypass at different temperatures. J Thorac Cardiovasc Surg 1994;107:1006-19. [PubMed]
- Kayatta MO, Chen EP. Optimal temperature management in aortic arch operations. Gen Thorac Cardiovasc Surg 2016;64:639-50. [Crossref] [PubMed]
- Gega A, Rizzo JA, Johnson MH, et al. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg 2007;84:759-66. [Crossref] [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28. [PubMed]
- Ergin MA, Uysal S, Reich DL, et al. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67:1887-90. [Crossref] [PubMed]
- Haverich A, Hagl C. Organ protection during hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2003;125:460-2. [Crossref] [PubMed]
- Urbanski PP, Lenos A, Bougioukakis P, et al. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg 2012;41:185-91. [PubMed]
- Zierer A, El-Sayed Ahmad A, Papadopoulos N, et al. Selective antegrade cerebral perfusion and mild (28°C-30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg 2012;144:1042-9. [Crossref] [PubMed]
- Tian DH, Wan B, Bannon PG, et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann Cardiothorac Surg 2013;2:148-58. [PubMed]
- Halkos ME, Kerendi F, Myung R, et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg 2009;138:1081-9. [Crossref] [PubMed]
- Tsai JY, Pan W, LeMaire SA, et al. Moderate hypothermia during aortic arch surgery is associated with reduced risk of early mortality. J Thorac Cardiovasc Surg 2013;146:662-7. [Crossref] [PubMed]
- Immer FF, Lippeck C, Barmettler H, et al. Improvement of quality of life after surgery on the thoracic aorta: effect of antegrade cerebral perfusion and short duration of deep hypothermic circulatory arrest. Circulation 2004;110:II250-5. [Crossref] [PubMed]
- Vallabhajosyula P, Jassar AS, Menon RS, et al. Moderate versus deep hypothermic circulatory arrest for elective aortic transverse hemiarch reconstruction. Ann Thorac Surg 2015;99:1511-7. [Crossref] [PubMed]
- Milewski RK, Pacini D, Moser GW, et al. Retrograde and antegrade cerebral perfusion: results in short elective arch reconstructive times. Ann Thorac Surg 2010;89:1448-57. [Crossref] [PubMed]
- Leshnower BG, Thourani VH, Halkos ME, et al. Moderate versus deep hypothermia with unilateral selective antegrade cerebral perfusion for acute type A dissection. Ann Thorac Surg 2015;100:1563-8. [Crossref] [PubMed]
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9. [Crossref] [PubMed]
- Strauch JT, Lauten A, Spielvogel D, et al. Mild hypothermia protects the spinal cord from ischemic injury in a chronic porcine model. Eur J Cardiothorac Surg 2004;25:708-15. [Crossref] [PubMed]
- Fernández Suárez F, Sánchez Burón J, Sánchez García V, et al. Cerebrospinal fluid drainage and deep systemic hypothermia with total absence of circulation for spinal cord protection during surgery on the thoracic aorta. Rev Esp Anestesiol Reanim 2001;48:192-5. [PubMed]
- Gong M, Ma WG, Guan XL, et al. Moderate hypothermic circulatory arrest in total arch repair for acute type A aortic dissection: clinical safety and efficacy. J Thorac Dis 2016;8:925-33. [Crossref] [PubMed]
- Pacini D, Pantaleo A, Di Marco L, et al. Visceral organ protection in aortic arch surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg 2014;46:438-43. [Crossref] [PubMed]
- Zhurav L, Wildes TS. Pro: topical hypothermia should be used during deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2012;26:333-6. [Crossref] [PubMed]
- Grocott HP, Andreiw A. Con: topical head cooling should not be used during deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2012;26:337-9. [Crossref] [PubMed]
- Svyatets M, Tolani K, Zhang M, et al. Perioperative management of deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2010;24:644-55. [Crossref] [PubMed]
- Mosca MS, Justison G, Reece TB. A Clinical protocol for goal directed cerebral perfusion during aortic arch surgery. Semin Cardiothorac Vasc Anesth 2016;20:289-97. [Crossref] [PubMed]
- Engelman R, Baker RA, Likosky DS, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: Clinical practice guidelines for cardiopulmonary bypass—temperature management during cardiopulmonary bypass. Ann Thorac Surg 2015;100:748-57. [Crossref] [PubMed]
- Reed H, Berg KB, Janelle GM. Aortic surgery and deep-hypothermic circulatory arrest: Anesthetic update. Semin Cardiothorac Vasc Anesth 2014;18:137-45. [Crossref] [PubMed]
- Coselli JS, LeMaire SA. Experience with retrograde cerebral perfusion during proximal aortic surgery in 290 patients. J Card Surg 1997;12:322-5. [PubMed]
- Safi HJ, Letsou G V, Iliopoulos DC, et al. Impact of retrograde cerebral perfusion on ascending aortic and arch aneurysm repair. Ann Thorac Surg 1997;63:1601-7. [Crossref] [PubMed]
- Estrera AL, Miller CC, Lee T-Y, et al. Ascending and transverse aortic arch repair: The impact of retrograde cerebral perfusion. Circulation 2008;118:S160-6. [Crossref] [PubMed]
- Ehrlich MP, Fang WC, Grabenwöger M, et al. Impact of retrograde cerebral perfusion on aortic arch aneurysm repair. J Thorac Cardiovasc Surg 1999;118:1026-32. [Crossref] [PubMed]
- Okita Y, Takamoto S, Ando M, et al. Mortality and cerebral outcome in patients who underwent aortic arch operations using deep hypothermic circulatory arrest with retrograde cerebral perfusion: no relation of early death, stroke, and delirium to the duration of circulatory arrest. J Thorac Cardiovasc Surg 1998;115:129-38. [Crossref] [PubMed]
- Tian DH, Wan B, Bannon PG, et al. A meta-analysis of deep hypothermic circulatory arrest alone versus with adjunctive selective antegrade cerebral perfusion. Ann Cardiothorac Surg 2013;2:261-70. [PubMed]
- Pochettino A, Cheung AT. Pro: Retrograde cerebral perfusion is useful for deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2003;17:764-7. [Crossref] [PubMed]
- Seco M, Edelman JJ, Van Boxtel B, et al. Neurologic injury and protection in adult cardiac and aortic surgery. J Cardiothorac Vasc Anesth 2015;29:185-95. [Crossref] [PubMed]
- Leshnower BG, Myung RJ, Kilgo PD, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg 2010;90:547-54. [Crossref] [PubMed]
- Khaladj N, Shrestha M, Meck S, et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg 2008;135:908-14. [Crossref] [PubMed]
- Kruger T, Weigang E, Hoffmann I, et al. GERAADA Investigators. Cerebral protection during surgery for acute aortic dissection type A: Results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43. [Crossref] [PubMed]
- Hagl C, Ergin MA, Galla JD, et al. Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg 2001;121:1107-21. [Crossref] [PubMed]
- Perreas K, Samanidis G, Thanopoulos A, et al. Antegrade or retrograde cerebral perfusion in ascending aorta and hemiarch surgery? A propensity-matched analysis. Ann Thorac Surg 2016;101:146-52. [Crossref] [PubMed]
- Guo S, Sun Y, Ji B, et al. Similar cerebral protective effectiveness of antegrade and retrograde cerebral perfusion during deep hypothermic circulatory arrest in aortic surgery: a meta-analysis of 7023 patients. Artif Organs 2015;39:300-8. [Crossref] [PubMed]
- Zierer A, Risteski P, El-Sayed Ahmad A, et al. The impact of unilateral versus bilateral antegrade cerebral perfusion on surgical outcomes after aortic arch replacement: a propensity-matched analysis. J Thorac Cardiovasc Surg 2014;147:1212-7; discussion 1217-8. [Crossref] [PubMed]
- Angeloni E, Benedetto U, Takkenberg JJ, et al. Unilateral versus bilateral antegrade cerebral protection during circulatory arrest in aortic surgery: a meta-analysis of 5100 patients. J Thorac Cardiovasc Surg 2014;147:60-7. [Crossref] [PubMed]
- Angeloni E, Melina G, Refice SK, et al. Unilateral versus bilateral antegrade cerebralpProtection during aortic surgery: An updated meta-analysis. Ann Thorac Surg 2015;99:2024-31. [Crossref] [PubMed]
- Malvindi PG, Scrascia G, Vitale N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact Cardiovasc Thorac Surg 2008;7:891-7. [Crossref] [PubMed]
- Deng J, Lei C, Chen Y, et al. Neuroprotective gases - Fantasy or reality for clinical use? Prog Neurobiol 2014;115:210-45. [Crossref] [PubMed]
- Homi HM, Yokoo N, Ma D, et al. The neuroprotective effect of xenon administration during transient middle cerebral artery occlusion in mice. Anesthesiology 2003;99:876-81. [Crossref] [PubMed]
- Kruger T, Hoffmann I, Blettner M, et al. Intraoperative neuroprotective drugs without beneficial effects? Results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2013;44:939-46. [Crossref] [PubMed]
- Schwer CI, Lehane C, Guelzow T, et al. Thiopental inhibits global protein synthesis by repression of eukaryotic elongation factor 2 and protects from hypoxic neuronal cell death. PLoS One 2013;8:e77258. [Crossref] [PubMed]
- Al-Hashimi S, Zaman M, Waterworth P, et al. Does the use of thiopental provide added cerebral protection during deep hypothermic circulatory arrest? Interact Cardiovasc Thorac Surg 2013;17:392-7. [Crossref] [PubMed]
- De Paulis R, Czerny M, Weltert L, et al. Current trends in cannulation and neuroprotection during surgery of the aortic arch in Europe. Eur J Cardiothorac Surg 2015;47:917-23. [Crossref] [PubMed]
- Zwerus R, Absalom A. Update on anesthetic neuroprotection. Curr Opin Anaesthesiol 2015;28:424-30. [Crossref] [PubMed]
- Hudetz JA, Iqbal Z, Gandhi SD, et al. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand 2009;53:864-72. [Crossref] [PubMed]
- Mitchell SJ, Merry AF, Frampton C, et al. Cerebral protection by lidocaine during cardiac operations: a follow-up study. Ann Thorac Surg 2009;87:820-5. [Crossref] [PubMed]
- Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 2015;386:1243-53. [Crossref] [PubMed]
- Erdoes G, Gerster G, Colucci G, et al. Prediction of post-weaning fibrinogen status during cardiopulmonary bypass: An observational study in 110 patients. PLoS One 2015;10:e0126692. [Crossref] [PubMed]
- Bhudia SK, Cosgrove DM, Naugle RI, et al. Magnesium as a neuroprotectant in cardiac surgery: A randomized clinical trial. J Thorac Cardiovasc Surg 2006;131:853-61. [Crossref] [PubMed]
- Chang JJ, Mack WJ, Saver JL, et al. Magnesium: potential roles in neurovascular disease. Front Neurol 2014;5:52. [Crossref] [PubMed]
- Afshari D, Moradian N, Rezaei M. Evaluation of the intravenous magnesium sulfate effect in clinical improvement of patients with acute ischemic stroke. Clin Neurol Neurosurg 2013;115:400-4. [Crossref] [PubMed]
- Mathew JP, White WD, Schinderle DB, et al. Intraoperative magnesium administration does notiImprove neurocognitive function after cardiac surgery. Stroke 2013;44:3407-13. [Crossref] [PubMed]
- Bilotta F, Gelb AW, Stazi E, et al. Pharmacological perioperative brain neuroprotection: a qualitative review of randomized clinical trials. Br J Anaesth 2013;110 suppl 1:i113-20. [Crossref] [PubMed]
- Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009;87:663-9. [Crossref] [PubMed]
- NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108-18. [Crossref] [PubMed]
- Guan XL, Wang XL, Liu YY, et al. Changes in the hemostatic system of patients with acute aortic dissection undergoing aortic arch surgery. Ann Thorac Surg 2016;101:945-51. [Crossref] [PubMed]
- Cappabianca G, Mariscalco G, Biancari F, et al. Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Crit Care 2016;20:5. [Crossref] [PubMed]
- Ortmann E, Besser MW, Sharples LD, et al. An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesth Analg 2015;121:26-33. [Crossref] [PubMed]
- Hanna JM, Keenan JE, Wang H, et al. Use of human fibrinogen concentrate during proximal aortic reconstruction with deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2016;151:376-82. [Crossref] [PubMed]
- Rahe-Meyer N, Solomon C, Hanke A, et al. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery. Anesthesiology 2013;118:40-50. [Crossref] [PubMed]
- Casati V, Sandrelli L, Speziali G, et al. Hemostatic effects of tranexamic acid in elective thoracic aortic surgery: a prospective, randomized, double-blind, placebo-controlled study. J Thorac Cardiovasc Surg 2002;123:1084-91. [Crossref] [PubMed]
- Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2011.CD001886. [PubMed]
- Makhija N, Sarupria A, Kumar Choudhary S, et al. Comparison of epsilon aminocaproic acid and tranexamic Acid in thoracic aortic surgery: clinical efficacy and safety. J Cardiothorac Vasc Anesth 2013;27:1201-7. [Crossref] [PubMed]
- Wilkey BJ, Weitzel NS. Anesthetic considerations for surgery on the aortic arch. Semin Cardiothorac Vasc Anesth 2016;20:265-72. [Crossref] [PubMed]
- Keenan JE, Benrashid E, Kale E, et al. Neurophysiological intraoperative monitoring during aortic arch surgery. Semin Cardiothorac Vasc Anesth 2016;20:273-82. [Crossref] [PubMed]
- Khoynezhad A, Celis R, Transcranial Doppler-guided selective antegrade cerebral perfusion during aortic debranching operation. J Thorac Cardiovasc Surg 2009;138:1029-30. [Crossref] [PubMed]
- Hayashida M, Sekiyama H, Orii R, et al. Effects of deep hypothermic circulatory arrest with retrograde cerebral perfusion on electroencephalographic bispectral index and suppression ratio. J Cardiothorac Vasc Anesth 2007;21:61-7. [Crossref] [PubMed]
- Orihashi K, Sueda T, Okada K, et al. Near-infrared spectroscopy for monitoring cerebral ischemia during selective cerebral perfusion. Eur J Cardiothorac Surg 2004;26:907-11. [Crossref] [PubMed]
- Olsson C, Thelin S. Regional cerebral saturation monitoring with near-infrared spectroscopy during selective antegrade cerebral perfusion: diagnostic performance and relationship to postoperative stroke. J Thorac Cardiovasc Surg 2006;131:371-9. [Crossref] [PubMed]
- Urbanski PP, Lenos A, Kolowca M, et al. Near-infrared spectroscopy for neuromonitoring of unilateral cerebral perfusion. Eur J Cardiothorac Surg 2013;43:1140-4. [Crossref] [PubMed]
- Harrer M, Waldenberger FR, Weiss G, et al. Aortic arch surgery using bilateral antegrade selective cerebral perfusion in combination with near-infrared spectroscopy. Eur J Cardiothorac Surg 2010;38:561-7. [Crossref] [PubMed]
- Stone JG, Young WL, Smith CR, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology 1995;82:344-51. [Crossref] [PubMed]
- Fassl J, Matt P, Eckstein F, et al. Transfusion of allogeneic blood products in proximal aortic surgery with hypothermic circulatory arrest: effect of thromboelastometry-guided transfusion management. J Cardiothorac Vasc Anesth 2013;27:1181-8. [Crossref] [PubMed]
- Mace H, Lightfoot N, McCluskey S, et al. Validity of thromboelastometry for rapid assessment of fibrinogen levels in heparinized samples during cardiac surgery: A retrospective, single-center, observational study. J Cardiothorac Vasc Anesth 2016;30:90-5. [Crossref] [PubMed]
- Vallabhajosyula P, Szeto WY, Desai N, et al. Type II arch hybrid debranching procedure. Ann Cardiothorac Surg 2013;2:378-86. [PubMed]
- Hogendoorn W, Schlösser FJ, Moll FL, et al. Thoracic endovascular aortic repair with the chimney graft technique. J Vasc Surg 2013;58:502-11. [Crossref] [PubMed]


