Prolonged air leak after video-assisted thoracic surgery lung cancer resection: risk factors and its effect on postoperative clinical recovery
Introduction
Prolonged air leak (PAL) is one of the major postoperative complications after lung surgery (1). According to previous studies, the incidence of PAL was approximately 10% (2,3). Several risk factors of PAL have been identified, including age, body mass index (BMI), surgeons and surgical site (4,5). Patients with PAL were also prone to have longer drainage time and higher incidence of postoperative complications (6-8). Therefore, those patients would have a prolonged length of stay (LOS) and increased medical costs than the patients without any postoperative complications (3).
Most of the previous studies on PAL were based on the data of patients underwent open thoracotomy (2-4). It is widely accepted that video-assisted thoracoscopic surgery (VATS) has shown tremendous advantages in lung surgery. Mountain evidence revealed that VATS had equivalent long term outcomes, less trauma, faster postoperative recovery and lower incidence of postoperative complications when comparing with open thoracotomy (9,10). Nowadays, VATS is becoming the more preferred approach for surgical treatment of lung diseases, especially in lung cancer surgery. However, there are still few studies on PAL after VATS lung cancer resection. We aimed to reveal the incidence and risk factors of PAL in lung cancer patients who underwent VATS major pulmonary resection, and to assess its effect on postoperative clinical recovery, including postoperative complications, postoperative length of stay (PLOS), and medical costs.
Methods
Patients
This study is a review of the Western China Lung Cancer Database which was established in late 2011, and prospectively collecting clinical data of lung cancer patients who underwent surgery in the Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China. Continuous patients who underwent VATS major pulmonary resection for lung cancer between January 2014 and December 2015 were studied. Patients who underwent wedge resection or pneumonectomy were excluded. Clinical data of those enrolled patients were obtained from the Western China Lung Cancer Database. The Institutional Review Board (IRB) of West China Hospital approved the use of these data (No. 2016-98). Patient’s informed consent was waived for this study.
Surgical technique
VATS lobectomy in our center was carried out with the “single-direction” technique as we previously described (11), which is also known as a “fissure-last” technique. Systematic nodal dissection was accomplished using the “non-grasping” method for all the patients (12). Finally, a 28 Fr chest tube was placed through the thoracoscopic port. The tube was removed when chest drainage was less than 300 mL per 24 hrs without any air leak, and complete re-expansion of the residual lung.
For patients with pleural adhesions, especially for those with complete pleural symphysis, adhesiolysis was carried out by constructing tunnels (13). Pleural adhesions were divided into three groups during surgery: (I) none pleural adhesion: without any adhesion; (II) minimal pleural adhesion: adhesiolysis within 30 minutes; and (III) extensive pleural adhesion: adhesiolysis for 30 minutes or longer.
Variables analyzed
PAL was defined as persistent air leak for more than 5 days after surgery. Baseline clinical parameters, including age, gender, smoking history, preoperative respiratory comorbidities, histological type, TNM stage, fissure development, pleural adhesion, surgical procedure, surgical site, and surgeon’s background, were analyzed for the risk factors of PAL. Surgeons were divided into two groups according to their background: (I) mainly focusing on lung and mediastinum diseases; (II) mainly focusing on esophageal diseases. Incidence of postoperative complications, PLOS and medical costs were analyzed to reveal the effect of PAL on patients’ clinical recovery. We mainly focused on postoperative respiratory and circulatory complications in this study, including pulmonary atelectasis, pneumonia, empyema, bronchopleural fistula, respiratory failure, pulmonary embolism, deep venous thrombosis, myocardial infarction, arrhythmia and heart failure. We also paid attention to urinary tract infection, unplanned reoperation after surgery and perioperative death (within 30 days after surgery).
Statistical analysis
Categorical variables were analyzed using χ2-test. The quantitative continuous variables were compared using the Student’s t-test or Mann-Whitney U test. All were two-tailed tests with the level of significance set at 0.05. Logistic regression was further performed to identify the risk factors of PAL out of the variables that were significant in univariate analyses. Odds ratio (OR) and 95% confidence interval (CI) were estimated using logistic regression. Unstandardized coefficients were estimated using linear regression analysis model. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) Version 22.0 for windows (Armonk, NY, USA: IBM Corp.).
Results
Patient characteristics
From January 2014 to December 2015, 1,175 continuous patients who underwent VATS pulmonary resection for lung cancer were identified from the Western China Lung Cancer Database. Among these patients, 190 of them were excluded, including 118 cases of wedge resection, 6 cases of pneumonectomy. Ultimately, a total of 1,051 patients were enrolled in this study. The patients were divided into two groups based on the absence or presence of PAL. Characteristics of these patients were listed in Table 1. The incidence of PAL was 10.6% (111/1,051). Ninety-four patients had preoperative respiratory comorbidities, of whom 16 (17.0%) had PAL. Among the 841 patients who had lung adenocarcinoma, 92 (10.9%) had PAL. The most common surgical procedure was lobectomy (n=886, 81.4%). And 99 of the patients (11.2%) who underwent VATS lobectomy had PAL after surgery.
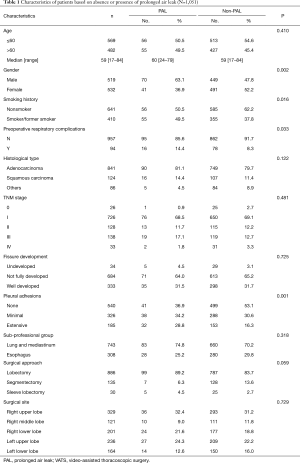
Full table
Risk factors for PAL
Gender (P=0.002), smoking history (P=0.016), preoperative respiratory comorbidities (P=0.033) and pleural adhesion (P=0.001) were identified as potential risk factors for PAL through univariate analysis (Table 1). Further multivariate analysis confirmed that pleural adhesion (P=0.004) was the only independent risk factor for PAL (Table 2). The incidence of PAL was significantly higher in patients with extensive pleural adhesion than the patients without pleural adhesion (17.3% vs. 7.6%; OR, 2.38; 95% CI: 1.43 to 3.95; P=0.001).

Full table
The effect of PAL on postoperative complications
Two hundred and ten (20.0%) patients presented with postoperative complications other than PAL. One died during the perioperative period. Fifty-seven (5.4%) patients had postoperative pneumonia (Table 3). The incidence of postoperative complications and postoperative pneumonia were 55.9% and 10.8% in PAL group, while 15.7% and 4.8% in non-PAL group, respectively. PAL was associated with higher risk of postoperative complications (OR, 6.77, 95% CI: 4.48 to 10.24; P=0.000) and postoperative pneumonia (OR, 2.41, 95% CI: 1.23 to 4.71; P=0.010). The incidence of other postoperative complications between PAL group and non-PAL group were insignificant.

Full table
The effect of PAL on PLOS and medical costs
The PLOS was significantly extended in PAL group than the non-PAL group (11.7±6.6 vs. 6.5±3.6 days; P=0.000). Total medical cost was 15.7% higher in the PAL group than non-PAL group (¥62,042.5±18,072.0 vs. ¥52,291.3±13,845.5, P=0.000) (Table 4).

Full table
Discussion
PAL is one of the most common postoperative complications in patients who underwent pulmonary resection, and it may affect the patient’s postoperative clinical recovery. The definition of PAL is divergent in different studies. The two most popular definitions of PAL are air leak >5 or ≥7 days after surgery (2,5,14,15). Current opinion prefers to consider an air leak as prolonged if it increased the length of an otherwise uncomplicated postoperative hospitalization (14). With the development of fast track surgery, the average PLOS of the patients who underwent lobectomy has been reduced to about 5 days (16). Therefore, we defined PAL as air leak more than 5 days after surgery. And the incidence of PAL with this definition is approximately 10% (2,3), which is consistent with our observation (10.6%).
A number of studies have assessed the risk factors of PAL. Male patients (5,17), patients with preoperative pulmonary dysfunction (such as emphysema and COPD) (8,18,19), pleural adhesion and less developed fissure (14,20) were prone to have PAL. The operation performed by different surgeons would also affect the incidence of PAL (4). In addition to the studies for the risk factors of PAL, a considerable number of studies have reported the postoperative effects of PAL. Patients with PAL were more likely to concurrent with pneumonia, empyema and other postoperative complications (3,4,7,17). However, most of the previous studies were based on the data of open thoracotomy, and the studied population included the patients with various pulmonary diseases. Lung cancer is the most common cancer-related deaths worldwide, and VATS is becoming the most popular surgical approach for lung cancer treatment. In this study, we focused on the risk factors of PAL and its effect on postoperative clinical recovery for lung cancer patients who underwent VATS major pulmonary resection.
Incomplete fissure was considered to increase the risk of PAL. However, our data showed that this is not an independent risk factor for PAL in this group of patients. This may be due to the “single-direction/fissure last” surgical technique, which divided the lung parenchyma lastly during surgery and avoided to mobilize the pulmonary artery through the lung fissures (11). Stamenovic and his colleagues have reported that the fissure last VATS lobectomy appears to be a superior technique to conventional VATS lobectomy in terms of preventing PAL (21). Based on our data, we also believe that the “single-direction” procedure may reduce the risk of air leak after surgery.
Okereke and his colleagues have demonstrated that different surgeons would also affect the incidence of PAL (4). We further analyzed whether the difference was associated with surgeons’ background. The surgeons were divided into two groups according to their background, one is mainly focusing on lung and mediastinum diseases, the others are mainly focusing on esophageal diseases. However, the occurrence of PAL between the two groups of surgeons was the same. With standardized training, surgeon’s background is not a risk factor of PAL.
Gender, smoking history and preoperative respiratory comorbidities were statistically significant in the univariate analysis, but these factors were not identified as independent risk factors in further multivariate analysis. The only risk factor of PAL in this group of patients was pleural adhesion. For the patients with pleural adhesion, especially for those with extensive adhesion, it is difficult to avoid lung parenchyma damage during adhesiolysis. And this would directly lead to the occurrence of air leak.
When studying the effect of PAL on postoperative clinical recovery, we found that the incidence of pneumonia and total complications was significantly higher in patients with PAL than those without. PAL has been found to increase various cardio-pulmonary complications in previous studies (3,4). We further compared the PAL group with non-PAL group on PLOS and medical costs. PAL prolonged PLOS and increased medical costs; this is because PAL patients require longer time of drainage, which may cause other insidious costs. On the other hand, PAL increased other complications, which may also prolong PLOS and increase medical costs. Varela and his colleagues have shown that PAL can lead to an increase of PLOS and medical costs, and giving the first detailed number of increased medical costs (3). We confirmed their findings and given the increased medical costs in China.
In this study, we firstly showed the risk factors and the postoperative effect of PAL in lung cancer patients who underwent VATS resection. In addition, the database we used has a rather larger sample size until now. Nonetheless, several limitations exist in this study. First, this is a review of a prospective database which allows the possibility of unobserved and therefore uncontrolled confounding factors. Thus, prospective multicenter observational studies are encouraged to acquire more detailed data. Second, the data of pulmonary function test and BMI was unavailable in some patients, and this limited our further study on analyzing some potential risk factors.
Conclusions
Intraoperative pleural adhesion could increase the risk of PAL. Lung cancer patients who had PAL after VATS had more postoperative complications, longer PLOS and higher medical cost.
Acknowledgements
Funding: This work was supported by the Key Science and Technology Program of Sichuan Province, China (2013SZ0005, 2014SZ0148) (to L Liu & W Li).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of West China Hospital (No. 2016-98). Informed consent was waived for this study.
References
- Hunt BM, Aye RW. Prolonged air leak after lung resection. Curr Respir Med Rev 2012;8:280-4. [Crossref]
- Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg 2010;90:204-9. [Crossref] [PubMed]
- Varela G, Jimenez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004;77:1205-10. [Crossref] [PubMed]
- Brunelli A, Xiume F, Al Refai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [Crossref] [PubMed]
- Stolz AJ, Schutzner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:334-6. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Klapper J, D'Amico TA. VATS versus open surgery for lung cancer resection: moving toward a minimally invasive approach. J Natl Compr Canc Netw 2015;13:162-4. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Liu C, Pu Q, Guo C, et al. Non-grasping en bloc mediastinal lymph node dissection for video-assisted thoracoscopic lung cancer surgery. BMC Surg 2015;15:38. [Crossref] [PubMed]
- Liu C, Pu Q, Liao H, et al. Constructing tunnels to troubleshoot complete pleural symphysis during video-assisted thoracic surgery. Video-assist Thorac Surg 2016;1:1-6. [Crossref]
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Gilbert S, Maghera S, Seely AJ, et al. Identifying Patients at Higher Risk of Prolonged Air Leak After Lung Resection. Ann Thorac Surg 2016;102:1674-9. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197-206. [Crossref] [PubMed]
- Cerfolio RJ, Bass CS, Pask AH, et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727-30. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Solidoro P, et al. Digital air leak monitoring after lobectomy for primary lung cancer in patients with moderate COPD: can a fast-tracking algorithm reduce postoperative costs and complications? J Cardiovasc Surg (Torino) 2010;51:429-33. [PubMed]
- Gómez-Caro A, Calvo MJ, Lanzas JT, et al. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg 2007;31:203-8. [Crossref] [PubMed]
- Stamenovic D, Bostanci K, Messerschmidt A, et al. Fissureless fissure-last video-assisted thoracoscopic lobectomy for all lung lobes: a better alternative to decrease the incidence of prolonged air leak? Eur J Cardiothorac Surg 2016;50:118-23. [Crossref] [PubMed]


