Intrapleural perfusion thermo-chemotherapy for pleural effusion caused by lung carcinoma under VATS
Introduction
Malignant pleural effusion (MPE), defined by the presence of malignant cells in the pleural space, reflects advanced lung cancer and is associated with significant morbidity (1). MPE is considered a crucial negative factor in reducing the patient’s quality of life (QOL) and survival (2). Many attempts to treat this disease, including surgery, have not been successful (3). Therefore, surgical therapy alone is generally not recommended. To improve prognosis, more extensive surgical procedures, such as pleuropneumonectomy and adjuvant chemotherapy, have been assessed, but no ‘gold standard’ for therapy has been established. Recently, the efficacy of pleural perfusion thermo-chemotherapy for MPE was reported (4,5); it consists of co-administration of cytotoxic drugs (such as cisplatin) and thermotherapy with thoracotomy (6). However, this method may not be suitable for patients with poor pulmonary function and cardiac complications. Indeed, the rate of complications in patients with limited pulmonary function is significantly higher in patients undergoing thoracoscopy (7,8). Therefore, in this study, we adopted IPTC for the video-assisted thoracoscopic surgery (VATS) approach in a minimally invasive manner for the treatment of pleural effusion caused by lung carcinoma.
Methods
From January 2004 to April 2013, fifty-four patients with MPEs caused by lung carcinoma but without distant metastasis underwent IPTC under VATS in our department. They were 45 to 75 years old, with a mean age of 64.9 years. All patients had been diagnosed with primary lung adenocarcinoma. The clinical stage of all patients was IV, since they had pleural metastasis (M1a) with no distant metastasis at the time of perfusion treatment. The patients underwent surgical pleural biopsy and received sequential perfusion treatment. This study was approved by the ethics committee of Hangzhou First People’s Hospital (No. 101-01 Ethics).
Therapy
Intrapleural perfusion thermo-chemotherapy (IPTC) under VATS was performed generally according to the conventional method without thoracotomy (9). The patients underwent general anesthesia, and were intubated by suitable double lumen endotracheal tubes; using one-lung ventilation, each patient was placed in the lateral decubitus position. First, a 10-mm port (port 1) was placed in the seventh intercostal space at the mid-axillary line. A second port was placed in the forth intercostal space at the anterior axillary line. Another port (port 3) was placed in the sixth intercostal space at the posterior axillary line. A thoracoscope was inserted through the first port, while the other two ports were utilized to perform biopsy and remove the fibrinous adhesions, thereby obtaining pleural biopsy tissues. Next, irrigation inlet (10 mm) and outlet (10 mm) tubes were inserted through ports 1 and 2, and connected to a standard extra-corporal circuit. The circuits were primed with 3,000 mL of saline solution; the pleural space perfusion flow rate was maintained between 800 and 1,000 mL/min. A temperature probe was inserted and placed in the pleural cavity via port 3. After confirming an intrapleural temperature of about 43 °C, cisplatin was administered at a total dose of 200 mg/m2. 500 mg methylprednisolone and 8 mg ondansetron were administered intravenously during IPTC to prevent pulmonary edema and vomiting, which are usually caused by thermotherapy. Once perfusion was completed, the solution in the thoracic cavity was completely removed, and a drainage tube placed via port 1. Blood pressure, heart rate, SpO2, and esophageal and rectal temperatures were monitored throughout the surgery. At the end of the perfusion, pleural biopsy was performed for histological analysis. Serum carcinoembryonic antigen (CEA) levels before and after IPTC were determined.
Histological analysis
Tumor tissues were collected from pleural effusions and nodules. One part was fixed in 10% formalin and paraffin embedded, and stained with H & E. The remaining part was fixed in 4% glutaraldehyde and cut into ultrathin sections for TEM.
Follow-up visit
Follow-up was performed every 3 months after hospital discharge.
Results
The temperature at the pleural surface was stabilized at 43 °C. The maximum temperature in the esophagus and rectum was less than 38.3 °C, despite the rise in pleural surface temperature.
Clinical outcomes and toxicities after IPTC under VATS
Efficacy was evaluated according to conventional criteria (10): (I) complete recovery (CR) was reflected by effusion clearance lasting for more than 4 weeks; (II) partial recovery (PR) indicated effusions reduced to less than 50%, lasting for more than 4 weeks; CR and PR represented effective treatment. After IPTC, 54 cases showed CR, and 2 had PR; pleural effusion was controlled in 100% of patients. Symptoms, including chest distress, shortness of breath, cough, and chest pain, were noticeably ameliorated. KPS scores were increased in 48 cases (88.9%) (Figure 1).
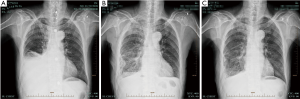
Changes in blood pressure and pulmonary artery pressure were mild. Two patients developed lung edema perioperatively, which was improved by induced diuresis, oxygen inspiration and steroid administration; 3 patients developed cardiac malfunction, which was ameliorated by heart stimulation and diuresis induction. No patient developed bone marrow suppression reaction with noticeable bleeding after treatment; no liver and kidney malfunctions were observed (Table 1).
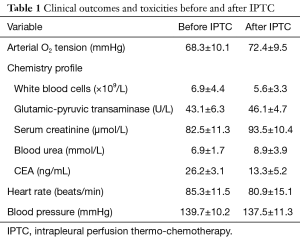
Full table
Baseline CEA levels were high preoperatively in all patients, with a mean value of 26.2±3.1 ng/mL. They markedly decreased 1 month after IPTC, showing a mean value of 13.3±5.2 ng/mL.
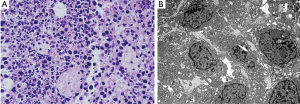
Morphological characteristics of tumor cell apoptosis were obvious under light microscopy and TEM, including pyknosis and karyorrhexis (Figure 2).
Follow-up visits persisted until October 2014 for all patients. A total of 40 patients survived for more than 1 year, with 19, 7, and 4 surviving over 2, 3, and 4 years, respectively. Median survival time was 21.7 months; the one-year survival rate was 74.1%.
Discussion
MPE, a common complication in patients with stage-IV lung cancer, can severely reduce the QOL and survival rate (11). Increased pleural fluid limits the mechanical dilation of the lungs, thereby affecting pulmonary and cardiac function; this usually results in severe shortness of breath and circulation failure. The International Association for the Study of Lung Cancer (IASLC) reported a 1-year survival rate of 36% in patients with carcinomatous pleuritis (12). Currently, the primary treatment for pleural fluid is local therapy (e.g., intrathoracic injection of chemotherapy drugs, solidifiers and bioactive agents). However, repeated operations often cause more pain to the patients and increase body exhaustion, and the reduction of thoracic effusion is not satisfactory. The traditional surgical therapy, including pleuropneumonectomy, often causes various complications and results in a high death rate; it is therefore not suitable for many patients (13).
It is a consensus in modern medicine that cancer cells would be killed when heated to 41.0–45.0 °C for dozens of minutes (14). Cancer cells are supported by abnormal capillaries and cannot store oxygen efficiently. The characteristics of cancer cells make them susceptible to heating (e.g., 42.0 °C), which considerably blocks or reduces their metabolism, as well as the activities of enzymes involved in cell division and DNA and RNA syntheses (15,16). Hyperthermia constitutes a highly effective tool for cancer treatment, particularly in combination with chemotherapy, radiotherapy or immunotherapy, which show synergetic effects (17-19). We perfused the pleural cavity with heated saline containing cisplatin through mechanical circulation, which resulted in the death of residual suspension cancer cells and low metastasis on the thoracic membrane, thereby blocking or reducing the pleural fluid.
In intrapleural thermo-chemotherapy, the chemotherapeutic drugs to be injected into the pleural cavity are diluted to about 3,000 mL and warmed to 43 °C. The drugs are quickly injected into the pleural cavity, and induce apoptosis of suspension cancer cells in the pleural cavity and low tumor metastasis. In this manner, the therapy can effectively reduce pleural effusion and increase survival.
In this study, we applied thoracoscopy under the VATS approach, which allows minimally invasive operation for lung decortication. Besides, the lung can be dilated after fiberboard removal; this distributes thermo-therapy more evenly within the pleural cavity, making it easier for the drugs to penetrate into the tissues, thereby enhancing the therapeutic effectiveness. Meanwhile, thoracoscopy can detect abnormalities in the lungs, pleural space and diaphragm, and can guide biopsy for pathological diagnosis and provide a pathological foundation for comprehensive treatment after surgery.
Overall, thermo-chemotherapy for lung cancer and MPE under video-assisted thoracoscope can help reach accurate pathological diagnosis and considerably reduce the effusion in a short period of time. Furthermore, this therapy is minimally invasive with few side effects, and can prolong patient survival. The above advantages of the current therapy strongly support broader application.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of Hangzhou First People’s Hospital (No. 101-01 Ethics).
References
- Morgensztern D, Waqar S, Subramanian J, et al. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol 2012;7:1485-9. [Crossref] [PubMed]
- Biaoxue R, Hui P, Wenlong G, et al. Evaluation of efficacy and safety for recombinant human adenovirus-p53 in the control of the malignant pleural effusions via thoracic perfusion. Sci Rep 2016;6:39355. [Crossref] [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [Crossref] [PubMed]
- Matsuzaki Y, Edagawa M, Shimizu T, et al. Intrapleural hyperthermic perfusion with chemotherapy increases apoptosis in malignant pleuritis. Ann Thorac Surg 2004;78:1769-72. [Crossref] [PubMed]
- Hofmann HS, Wiebe K. Cytoreductive surgery and hyperthermic intrathoracic chemotherapy perfusion. Chirurg 2010;81:557-62. [Crossref] [PubMed]
- Ishibashi H, Kobayashi M, Takasaki C, et al. Interim results of pleurectomy/decortication and intraoperative intrapleural hyperthermic cisplatin perfusion for patients with malignant pleural mesothelioma intolerable to extrapleural pneumonectomy. Gen Thorac Cardiovasc Surg 2015;63:395-400. [Crossref] [PubMed]
- Migliore M, Calvo D, Criscione A, et al. Cytoreductive surgery and hyperthermic intrapleural chemotherapy for malignant pleural diseases: preliminary experience. Future Oncol 2015;11:47-52. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Matsuzaki Y, Shibata K, Yoshioka M, et al. Intrapleural perfusion hyperthermo-chemotherapy for malignant pleural dissemination and effusion. Ann Thorac Surg 1995;59:127-31. [Crossref] [PubMed]
- Kang M, Zhou L, Lin P. Treatment of pleural effusion caused by lung carcinoma with circular intrapleural hyperthermic perfusion and its mechanism. Zhonghua Yi Xue Za Zhi 2001;81:1176-9. [PubMed]
- Yokoi K, Matsuguma H. Surgical treatment of lung cancer with carcinomatous pleuritis. Nihon Geka Gakkai Zasshi 2013;114:196-200. [PubMed]
- Fukui T, Yokoi K. The role of surgical intervention in lung cancer with carcinomatous pleuritis. J Thorac Dis 2016;8:S901-7. [Crossref] [PubMed]
- Go T, Misaki N, Matsuura N, et al. Role of surgery in multi-modality treatment for carcinomatous pleuritis in patients with non-small cell lung cancer. Surg Today 2015;45:197-202. [Crossref] [PubMed]
- Kim HC, Kim E, Jeong SW, et al. Magnetic nanoparticle-conjugated polymeric micelles for combined hyperthermia and chemotherapy. Nanoscale 2015;7:16470-80. [Crossref] [PubMed]
- Hu R, Ma S, Li H, et al. Effect of magnetic fluid hyperthermia on lung cancer nodules in a murine model. Oncol Lett 2011;2:1161-4. [PubMed]
- Speit G, Schütz P. Hyperthermia-induced genotoxic effects in human A549 cells. Mutat Res 2013;747-748:1-5. [Crossref] [PubMed]
- Zhao P, Jiang H, Su D, et al. Inhibition of cell proliferation by mild hyperthermia at 43˚C with Paris Saponin I in the lung adenocarcinoma cell line PC-9. Mol Med Rep 2015;11:327-32. [PubMed]
- Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487-97. [Crossref] [PubMed]
- Saga T, Sakahara H, Nakamoto Y, et al. Enhancement of the therapeutic outcome of radio-immunotherapy by combination with wholebody mild hyperthermia. Eur J Cancer 2001;37:1429-34. [Crossref] [PubMed]


