Bicuspid aortic valve syndrome: a multidisciplinary approach for a complex entity
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease with an incidence in the general population of between 0, 6% and 2% (1). Far from being just a valvulopathy it is a heterogeneous and complex disease with great clinical impact. It can lead to early severe valve dysfunction, bacterial endocarditis and even acute aortic dissection.
Different phenotypes related to valve morphology have been described, also distinct genetics alterations are widely accepted and the pathogenesis of associated aortic dilatation is not completely clear (2). Echocardiography, mainly transthoracic is the main diagnostic tool for BAV but transesophageal and 3-dimensional echocardiography are sometimes necessary to confirm diagnosis. Otherwise, multimodality imaging, using both multidetector computed tomography and magnetic resonance imaging are useful not only to study the ascending aorta but also to complete the evaluation of the valve anatomy (3,4).
During the last years there has been a growing interest for BAV disease, which is called also BAV syndrome and BAV aortopathy and more questions than answers have appeared (5).
As a result of that concern, we are progressively knowing more and more of the heterogeneity of this entity, being that interest the reason for this manuscript in which we will try to review the more relevant aspects related to this cluster of diseases called BAV syndrome.
Different morphotypes in BAV disease: from valve morphology to aortic dilatation
Instead of the normal three leaflets or cusps, BAV only presents two of them, usually of unequal size. The fusion of two cusps forms a ridge, called a raphe, the presence and position of which contributes to the variability of morphology.
As an example of the diversity that characterizes this congenital heart disease, different classifications attending to the presence and position of the raphes, the spatial position of the cusps and the commissures have been published. The main classifications of BAV morphology have been recently resumed by Longobardo et al. (2). Since Roberts, that based its classification in a study of autopsy cases, to Kang using multidetector computed tomography, different systems have been proposed, like the one by Brandenburg et al. based on commissures and raphe position according to 2-dimensional echocardiography (3,6,7). One of the most reproducible classifications is the one proposed by Sabet et al. bases on the presence and position of the raphes. This study was made in surgically excised valves and authors described different phenotypes based on raphe position (right-left, right-non coronary and left-non coronary) (8). Other classifications like the one described by Sievers and Schmidtke based on number of raphes, spatial arrangement of the cusps and functional status of the valve are however more complicated (9) (Figure 1).
Anyway the most common BAV phenotype and typical pattern is the fusion of the right and left cusps, resulting in an anteroposterior leaflet orientation, followed by the fusion of the right and non-coronary cusps (2,10). Otherwise, pattern of aortic dilatation is also diverse. Prevalence of aortic dilatation has a large variation depending on the study population, region of the aorta considered and diagnostic techniques and ranges from 20% to 84% (10,11). Again and as an example of heterogenicity, different classifications have been proposed to describe the aortopathy. In a recent manuscript by Verma et al., authors make a perfect review about aortic dilatation patterns in BAV which pathogenesis is still unclear. Three patterns have been proposed: type I the most common, is the dilatation of tubular ascending aorta with mild to moderate root dilatation, type II corresponds to isolated involvement of the tubular ascending aorta with relative sparing of root and finally type III involves isolated root dilatation (10) (Figure 2).
The relevance of BAV aortopathy is clear and it is well known that these patients have an increased risk of dissection however the pathogenesis of this aortic disease is still a matter of debate and genetic and hemodynamic factors have been proposed.
Pathogenesis of BAV and aortic dilatation
BAV and aortopathy: a close relationship
There is a feared risk of a progressive aortic dilatation associated to BAV. Although the tubular ascending aorta is the most commonly affected segment (60–70%), the root, aortic arch or even, although less frequently, the descending aorta may also be involved (12).
In comparison with three-leaflet aortic valves, BAVs present an increased risk of thoracic aortic aneurysm (TAA) and dissection (TAAD). As a result and due to the prevalence of BAV, the most common congenital heart defect, the general incidence of aneurysm formation entails an actual concern (12).
BAV patients have an increased risk of requiring aortic surgery, with a cumulative 25-year risk of 25% for aortic surgery and a 53% risk of receiving valve replacement (13).
Although the risk of acute complications (i.e., rupture and dissection) is still the object of controversies; an approximately eight times increased risk compared to general population has been reported (13).
One of the largest available studies, with 416 BAV patients and an average of 16 years of follow-up, reported an over 25% risk of aortic aneurism at 25 years after initial diagnosis, with an incidence of 85 per 10,000 patient-years. This would suppose an increased risk of developing aortic aneurysm, 80 times greater than in general population (14). Moreover, due to the dissection incidence increased nearly 15 times in those with aortic dilatation over 45 mm, an association between dilatation and dissection was also described (14,15).
Although the most commonly dilated segment in BAV patients is the tubular ascending aorta, in up to 25% of its dilated aortas is the aortic root the one affected, with sinus of Valsalva dilatation (16,17). This so called “root phenotype” has been associated with male sex, right-left cusp fusion and aortic insufficiency that may lead to a faster root and tubular ascending aorta dilatation and higher dissection (18,19).
It must be highlighted that it is important to control periodically the entire thoracic aorta in BAV patients, because aortic dissections can involve both the ascending and descending aorta (20). Moreover, after ascending aorta repair, it is also mandatory to periodically control the unrepaired aorta (16).
The prevalence of aortic dilatation increases with age (21). In tubular ascending aorta, an approximately 0.5 mm/year growing rate has been described, apparently without any influence depending on which were the fused cusps (17).
Syndromic conditions associated to BAV, like Loeys-Dietz and Turner syndromes, also represent an additional risk factor for dissection for aortic dissection risk (12).
Moreover, coarctation of the aorta (16) and uncontrolled hypertension may also increase the aortic dissection risk.
In summary, there is an aortopathy associated to BAV, presenting as an aortic dilatation in which the whole aorta may be affected. Thus, complete aorta must be periodically controlled, regardless of whether it had an initial normal size, the aortic valve function or underwent previous surgery (16).
Aortic dilatation: intrinsic or induced development?
There are two different theories to explain the both pathogenesis and perpetuation of aortic dilatation in BAV. On the one hand it is believed to be secondary to abnormal aorta fluid hemodynamics. On the other hand, an individual predisposition secondary to inherited factors also plays an important role (16).
It is known that development of the semilunar valves is intimately linked to outflow tract septation and ascending aorta/aortic arch remodeling. As a result, an alteration in the common pathway may explain the coexistence of both abnormalities. Neural crest cells are implicated in the semilunar valve development, through the vascular smooth cells of the ascending aorta formation. In mice, when Notch signaling is disrupted, the neural crest cell pattern is disturbed, associating aortic valves with bicuspid-like morphology (22).
Otherwise, many authors support the abnormal hemodynamic hemodynamic mechanism. Aortopathy is thought to be secondary to the eccentric and turbulent flow through the BAV that produces an abnormal hemodynamic stress on the aortic wall (2). Dysfunctional BAV evaluated by echocardiogram has already been associated to aortic dilatation (14). However, recently, it has been described that regardless of whether or not there is dysfunctional BAV by echocardiogram (23) both the hemodynamics and aortic wall stress are affected. This supports the hemodynamic etiopathogenic mechanism, but also substantiates that even an apparently normal BAV in echocardiogram is, in fact, intrinsically dysfunctional (2).
Thus, the risk of segmental aneurysm formation may be determined depending on the direction of the abnormal produced flow jet (2). Moreover, this abnormal flow may be determined by the fusion BAV phenotype, being right-left cusp fusion associated with root dilatation and right-non cusp fusion with tubular ascending and arch dilatation (2,24).
However, this theory cannot explain the tendency toward aortic dilatation in non BAV, first-degree relatives of BAV patients nor why aortic valve replacement does not halt aortic dilatation progression in BAV (25).
Genetic aspects of BAV and associated cardiovascular malformations: again diversity
BAV often co-occurs with other congenital heart defects. BAV is included in the spectrum of left-sided heart defects integrated by aortic stenosis, coarctation of the aorta, mitral valve abnormalities, Shone’s complex and hypoplastic left heart. Moreover, more than 50% of people with coarctation of the aorta have BAV (26). Even though BAV patents are usually non-syndromic, BAV can also represent a characteristic of connective tissue disorders (such as Marfan or Loeys-Dietz syndromes) and other syndromes such as Turner and Williams.
Traditionally BAV has been considered a sporadic malformation. However, reports of familial association support an underlying genetic abnormality. The most common forms of familial BAV appear to occur in an autosomal-dominant pattern but with incomplete penetrance and variable expressivity (27). Moreover, BAV has been observed in case reports of monozygotic twins (28). However, the male predominance and the association of BAV with Turner syndrome point to a possible X-linked inheritance (29).
Screening of first-degree family members of BAV patients is recommended by the consensus guidelines, due to its relatively high heritability rates (30), with the potential to identify a 9% unknown BAV in these first-degree relatives (29). Moreover, is should be reminded that BAV first-degree relatives, even with normal tricuspid aortic valves, may develop artic dilatation (25).
Although the heritability of BAV is well known, the genetic causes of BAV and associated diseases are still waiting to be defined. The inability to identify simple mendelian loci with whole-exome approaches may suggest a polygenic influence rather than simply incomplete penetrance (31).
In cardiac valve morphogenesis there are multiple signaling pathways involved, such as members of the TGF-β superfamily, VEGF, Notch, Wnt/β-catenin, bx20, and Gata4 (32). Many other genes, including genes involved in connective tissue disorders, cell signaling, and the extra-cellular matrix, have also been associated to BAV. The alteration of these pathways may produce or be related to BAV and studies developed in mouse models are contributing to clarify the new BAV candidate genes role (32).
Nowadays, NOTCH1 remains the only proven candidate gene and has been associated with both familial and sporadic BAV (33). Mutations in NOTCH1 were reported to segregate with affected patients in a five-generation European-American family and, in addition, in a second and smaller Hispanic family (33). Following this study, mutations in NOTCH1 were described among patients with sporadic BAV and concomitant TAAs in several subsequent studies (34).
A missense mutation in transforming growth factor-beta receptor 2 gene (TGFBR2) was found in a patient with BAV and aortic aneurysm (35), but no mutations were found in either TGFBR1 or TGFBR2 in patients with familial and sporadic BAV disease (34). Otherwise, about 12% of BAV patients with inherited TAA carry mutations in the ACTA2 gene (36).
In the KCNJ2 gene, some pathogenic variants have been found associated with Andersen syndrome, in which BAV can be one of its cardiovascular malformations. Nevertheless, this gen has not been linked to isolated BAV (37).
An association between Fibrillin 1gen variants and BAV with Marfan syndrome (38) has been found, as well as in BAV patients with aortic dilatation without Marfan syndrome and other type 1 fibrillinopathies (39).
The nitric oxide synthase (NOS) pathway is important in the development of the trileaflet aortic valve. Nitric oxide has been shown to have a crucial role in post developmental vascular remodeling and angiogenesis, and its importance in the development of the aortic valve was demonstrated by NOS3 null mice models (40) that showed a broad spectrum of cardiac malformations, including BAV, all from the fusion of the right and noncoronary leaflets (2). Recently, a novel mutation in the NKX2.5 gene, which encodes a protein involved in the activation of NOS promoters, was identified in BAV family (38). Functional assays revealed that the mutant protein had no transcriptional activity and abolished the synergistic transcriptional activation between NKX2.5 and GATA5, another transcription factor crucial for aortic valvular morphogenesis. In addition, rare genetic variants in the GATA5 gene, a member of the GATA family of transcription factors crucial for heart morphogenesis, were reported in several patients with BAV, pointing to a possible role for GATA5 in the pathogenesis of BAV (41).
Finally, multiple other common genetic variants [e.g., angiotensin-converting enzyme (ACE) and matrix metalloproteinase (MMP) polymorphisms] may act as modifier genes in the pathogenesis of BAV aortopathy, contributing to the variability of clinical phenotypes (42,43).
Imaging techniques for BAV disease: from diagnosis to follow-up
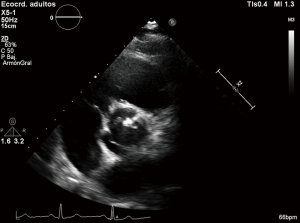
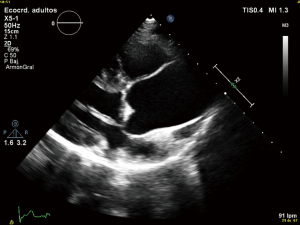
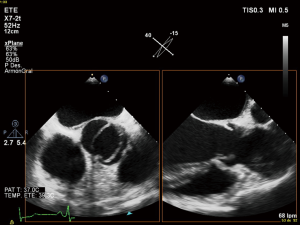
First diagnostic approach for the initial assessment of BAV is transthoracic echocardiogram. It allows not only the evaluation of the valvulopathy but also the assessment of thoracic aorta. If transthoracic acoustic window is appropriate, measures of aortic root, tubular ascending aorta, aortic arch and descending aorta can be easily made.
However, things are not always so simple. Acoustic window can be not optimal and the measures by echocardiography can be not perpendicular to the long axis of the tubular aorta which consequently, can lead to erroneous dimensions (16,44). Magnetic resonance imaging (MRI) and computed tomography (CT) utilize a “double oblique” measurement technique that provides a cross-sectional diameter that is perpendicular to the longitudinal or flow axis. This is the reason why, some authors recommend that aortic diameters >40 mm measured by echocardiography should be confirmed by MRI or CT. If measurements are comparable and reproducible between techniques, then follow up measurements may be obtained by echocardiography (16). Transesophageal echocardiography can be also useful in some cases. MRI, otherwise, if preferable to CT because it is not associated with radiation exposure (Figures 3,4). Recommendations for aortic imaging techniques to determine the presence and progression of thoracic aortic disease are clear in clinical guidelines. Of course, patients must be follow up according to the severity of the valvulopathy and anyway, yearly follow up is recommend in patients with aortic root or ascending aorta >40 mm (16,44).
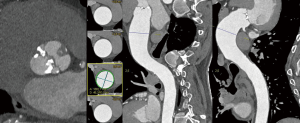
BAV disease in the clinical guidelines
The ESC guidelines in its documents “Guidelines on the management of valvular heart disease 2012” (VHD 2012) and the “2014 ESC Guidelines on the diagnosis and treatment of aortic diseases” (AD 2014) specifically contemplate BAV disease (30,45).
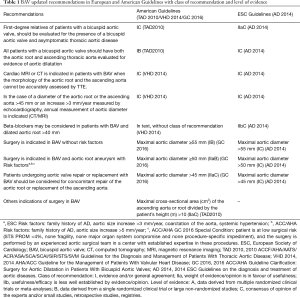
First, VHD 2012 exposed surgery indications in BAV with aortic root disease (whatever the severity of valvular disease); with threshold of aortic root or ascending aortic dilatation in 55 mm or greater (IIaC) and in 50 mm or greater and an additional risk factor for dissection-family history of aortic dissection, aortic growth rate >3 mm/year, coarctation of the aorta, systemic hypertension (IIaC). In text, without class of recommendation, authors indicated aortic root surgery in patients undergoing aortic valve repair or replacement with BAV and maximal aortic diameter >45 mm (30).
Later, AD 2014 dedicated a specific section to this entity. Indicating the recommendations of screening in first-degree relatives (IIaC), imaging test, follow-up and medical (IIbC) or surgery treatment (IC) (Table 1). The threshold of aortic root or ascending aortic dilatation were the same as in VHD 2012; but the class of recommendations and level of evidence were different (45) (Table 1).
Full table
Two guidelines from the ACCF/AHA and collaborating societies evaluated the BAV: in two documents in 2010 and 2014 (TAD 2010 and VHD 2014) (44,46). Nevertheless, the two guidelines differ with regard to the recommended threshold of aortic dilatation for surgical intervention. So ACC/AHA draft a statement of clarification for both guidelines: The “2016 ACC/AHA Guideline Clarification: Surgery for Aortic Dilatation in Patients With Bicuspid Aortic Valves” (GC 2016) (47).
The TAD 2010, inscribed an special paragraph with recommendations of screening in first-degree relatives (IC), imaging test (IB) and surgery treatment with the threshold of maximal aortic diameter in 50mm for patients without risk factor (IC) (44) (Table 1).
In VHD 2014, the authors indicated recommendations for diagnosis test, follow up and medical treatment (similar to ESC PG). By the aortic surgery indications, the threshold of maximal aortic diameter was in 55 mm for patients without risk factors (IB) (5 mm different to TAD 2010) and 50 mm with risk factors (Family history of AD, aortic size increase >5 mm/year) (IIaC). Like in ESC PG, patients undergoing aortic valve repair or replacement with BAV should be considered for concomitant repair of the aortic root or replacement of the ascending aorta if maximal aortic diameter >45 mm (IIaC) (47). Recently GC 2016 draft a statement of clarification for previous guidelines, recommending aortic surgery in BAV with maximal aortic diameter ≥55 mm (IB), ≥50 with risk factors (IIaB) or low surgical risk and >45 mm in associated valvular procedure (IIaC) (47) (Table 1).
In conclusion, the last recommendations for screening, imaging test, follow-up, medical treatment and surgery indications are very similar in European and American practice guidelines (Table 1).
Special aspects in BAV disease: medical treatment, pregnancy and sports participation
What about medical treatment
About medical therapy, a good control of high blood pressure is mandatory in patients with aortopathy. Extrapolation from Marfan disease, treatment with Beta Blocker has also been introduced in BAV-aortopathy. This way, both American and European Guidelines established that Beta-blockers may be considered in patients with BAV and dilated aortic root (>40 mm) (Class IIb in ECS guidelines AD 20014) (30,46). A Canadian clinical trial on Beta Blockers and Angiotensin Receptor Blockers in Bicuspid Aortic Valve Disease Aortopathy (BAV Study) has just finished and probably will resolve doubts about the effectiveness of these drugs in the progression of aortopathy (10,16).
What about sports participation
In athletes with Marfan syndrome and other similar entities like BAV aortopathy a matter of special concern is acute aortic dissection as a cause of sudden death. For this reason, patients with aortic disease should receive proper information about safe sports activities.
The Scientific statement recently published from the American Heart Association by Braverman et al. has perfectly resumed the recommendations for BAV patients and other aortic conditions. Of course, the function of the valve (whether stenotic or regurgitant) is important in determining participation recommendations and athletes with a dilated aortic root or ascending aorta (>45 mm) should not participate in any competitive sports (48).
What about pregnancy
Pregnancy increases hemodynamic stress over the vascular wall causing, at the same time, structural and histological changes that can contribute to progressive aortic dilatation or even dissection in women with aortopathies (49,50). Otherwise patients with BAV and severe aortic stenosis are also at risk of complications.
For this reason, an appropriate preconception counselling and evaluation with a multidisciplinary approach is crucial for these patients.
In case of aortopathy, recommendations for surgery prior to pregnancy are clear in clinical guidelines, this way, ESC guidelines for cardiovascular disease during pregnancy reflect that prophylactic surgery pre-pregnancy should be considered if aortic diameter is >50 mm (51). Besides, and depending on the aortic diameter, patients should be monitored by echocardiography at 4–12-week intervals throughout the pregnancy and 6 months post-partum. In the study published by McKellar in pregnant women with BAV no aortic complications were observed in 82 patients, however only 8% had and aortic diameter >40 mm (52).
Surgical aspects. Is there a place for repair? And is there a place for transcatheter aortic valve implantation (TAVI)?
Surgical aspects: a place for repair?
In BAV patients, clinical manifestations of possible aortic disease occur at an earlier age than in the tricuspid valves. Prosthetic valve implantation is the treatment of choice in patients with BAV disease and aortic stenosis, with a 15-year survival rate of 78% (53,54); however, being young patients with prolonged life expectancy are more likely to present complications related to prosthetic valves, such as the need for anticoagulation, thrombosis and thromboembolism in mechanical prostheses, degeneration in biological prostheses and infection in both. The incidence of complications, both mechanical and biological, reaches 3–5% per year (55) and the cumulative risk of complications is 50% at 10 years (55,56).
Patients with BAV diseased and aortic regurgitation have two therapeutic alternatives, valvular prosthesis implantation and aortic valve repair. Valve repair reduces the incidence of valve related complications to 1.6% (57), and is an alternative to consider because long-term durability is good 81% at 10 years (55).
The techniques described in BAV repair are multiple and are used according to each patient. There are techniques on the leaflets (central plicature, reconstruction of leaflets with pericardium, resuspension of leaflets), annulus techniques (annuloplasty with suture or ring), and if root and ascending aorta dilatation is to be treated, remodeling or reimplantation techniques are used.
There are several aspects of repair that can influence durability. (I) The orientation of the commissures: between 160°–180°, the stress of the leaflets is lower and improves durability (58). (II) The use of external material is associated with less durability, for two reasons: the external material itself degenerates before and the use of external material implies a greater alteration of the valve, so the repair is more complex. The heterologous pericardium is a very suitable material to use (59). (III) Annulus stabilization: an aorto-ventricular junction >28 mm contributes to repair failure (60). Stabilization with annuloplasty with suture (60) or with ring (61) provides good long-term results. To obtain good durability over time, a good coaptation of leaflets, aortic annulus stabilization and aortic root stabilization should be achieved.
An important factor in the surgical treatment of BAV is dilatation of the root and/or ascending aorta. There are several factors involved, both genetic and related to the type of leaflet fusion and valvular opening form. BAV stenosis associated with root dilatation should be treated with a valve tube. If there is aortic root dilatation and valvular regurgitation, together with the aortic repair we will associate a root technique, valvular reimplantation (62) or aortic remodeling (63). Valve reimplantation provides stability of the annulus by itself with good durability, 100% free of reoperation at 6 years (64), but in aortic remodeling with BAV, annuloplasty (60) has to be associated, obtaining at 10 years 89% free of reoperation.
Aortic repair in BAV has an increasingly important role, with good long-term durability, reduction of complications related to valvular disease, elimination of anticoagulation in young patients and young adults and improvement of the quality of life with a lower feeling of illness perceived by patients. Aortic repair is a demanding surgery for the surgeon and the patient; it should be assessed if its potential benefit outweighs the risks. In order to minimize risks and obtain good results valvular repair should always be performed in an experienced medical center.
Is there a place for TAVI?
BAV is generally considered a contraindication for TAVI. A poor stability of the prosthetic valve leading with a risk of stent-valve displacement, distortion or malfunctioning is feared, in addition to the risk of residual aortic regurgitation. Nevertheless, nowadays, patients with BAV and severe aortic stenosis who are high –risk surgical candidates are being increasingly referred for TAVI consideration. Although a systematic review supports a potential role for TAVI in BAV non-surgical patients, further studies about TAVI in BAV’s patients are required. Acceptable outcomes of TAVI may depend on some anatomical features such as heavily calcification valves and the presence of an enlarged root (65,66).
Future directions
Many questions are still unsolved in BAV. Calcium deposition and fibrosis are generally accelerated and patients develop severe stenosis or regurgitation earlier than patients with normal tricuspid aortic valves. Different mechanisms are proposed: on the one hand, genetic predisposition and on the other, local stress in the valve tissue secondary to the bicuspid morphology which alters blood flow; both can contribute to the early mineralisation of the leaflets. Further researches with multidisciplinary approaches are necessary in order to know more and better this complex entity and all over the place, in order to stop progression of the disease in early stages (67).
Acknowledgements
To all those who have made their vocation their profession.
Footnote
Conflicts of Interest: Dr. C Morís is Proctor for Medtronic. The other authors have no conflicts of interest to declare.
References
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000;83:81-5. [Crossref] [PubMed]
- Longobardo L, Jain R, Carerj S, et al. Bicuspid Aortic Valve: Unlocking the Morphogenetic Puzzle. Am J Med 2016;129:796-805. [Crossref] [PubMed]
- Kang JW, Song HG, Yang DH, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging 2013;6:150-61. [Crossref] [PubMed]
- Wassmuth R, von Knobelsdorff-Brenkenhoff F, Gruettner H, et al. Cardiac magnetic resonance imaging of congenital bicuspid aortic valves and associated aortic pathologies in adults. Eur Heart J Cardiovasc Imaging 2014;15:673-9. [Crossref] [PubMed]
- Nistri S, Giusti B, Pepe G, et al. Another piece in the puzzle of bicuspid aortic valve syndrome. Eur Heart J Cardiovasc Imaging 2016;17:1248-9. [Crossref] [PubMed]
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970;26:72-83. [Crossref] [PubMed]
- Brandenburg RO Jr, Tajik AJ, Edwards WD, et al. Accuracy of 2-dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: echocardiographic-anatomic correlation in 115 patients. Am J Cardiol 1983;51:1469-73. [Crossref] [PubMed]
- Sabet HY, Edwards WD, Tazelaar HD, et al. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999;74:14-26. [Crossref] [PubMed]
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33. [Crossref] [PubMed]
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920-9. [Crossref] [PubMed]
- Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation 2009;119:880-90. [Crossref] [PubMed]
- Michelena HI, Prakash SK, Della Corte A, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014;129:2691-704. [Crossref] [PubMed]
- Thanassoulis G, Yip JW, Filion K, et al. Retrospective study to identify predictors of the presence and rapid progression of aortic dilatation in patients with bicuspid aortic valves. Nat Clin Pract Cardiovasc Med 2008;5:821-8. [Crossref] [PubMed]
- Michelena HI, Khanna AD, Mahoney E, et al. Incidence of aortic complications in patients with bicuspid aortic valves JAMA 2011;306:1104-12. [Crossref] [PubMed]
- Coady MA, Rizzo JA, Hammond GL, et al. Surgical intervention criteria for thoracic aortic aneurysms: a study of growth rates and complications. Ann Thorac Surg 1999;67:1922-6. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Prakash SK, et al. Bicuspid aortic valve aortopathy in adults: Incidence, etiology, and clinical significance. Int J Cardiol 2015;201:400-7. [Crossref] [PubMed]
- Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging 2013;6:1301-10. [Crossref] [PubMed]
- Detaint D, Michelena HI, Nkomo V, et al. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart 2014;100:126-34. [Crossref] [PubMed]
- Girdauskas E, Disha K, Raisin HH, et al. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012;42:832-7. [Crossref] [PubMed]
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317-25. [Crossref] [PubMed]
- Sievers HH, Stierle U, Mohamed SA, et al. Toward individualized management of the ascending aorta in bicuspid aortic valve surgery: the role of valve phenotype in 1362 patients. J Thorac Cardiovasc Surg 2014;148:2072-80. [Crossref] [PubMed]
- Jain R, Engleka KA, Rentschler SL, et al. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest 2011;121:422-30. [Crossref] [PubMed]
- Hope MD, Hope TA, Crook SE, et al. 4D flow CMR in assessment of valve-related ascending aortic disease JACC Cardiovasc. Imaging 2011;4:781-7. [Crossref] [PubMed]
- Bissell MM, Hess AT, Biasiolli L, et al. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging 2013;6:499-507. [Crossref] [PubMed]
- Loscalzo ML, Goh DL, Loeys B, et al. Familial thoracic aortic dilation and bicommissural aortic valve: a prospective analysis of natural history and inheritance. Am J Med Genet A 2007;143A:1960-7. [Crossref] [PubMed]
- Roos-Hesselink JW, Schölzel BE, Heijdra RJ, et al. Aortic valve and aortic arch pathology after coarctation repair. Heart 2003;89:1074-7. [Crossref] [PubMed]
- Braverman AC, Güven H, Beardslee MA, et al. The bicuspid aortic valve. Curr Probl Cardiol 2005;30:470-522. [Crossref] [PubMed]
- Brown C, Sane DC, Kitzman DW. Bicuspid aortic valves in monozygotic twins. Echocardiography 2003;20:183-4. [Crossref] [PubMed]
- Laforest B, Nemer M. Genetic insights into bicuspid aortic valve formation. Cardiol Res Pract 2012;2012:180297. [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Martin LJ, Pilipenko V, Kaufman KM, et al. Whole exome sequencing for familial bicuspid aortic valve identifies putative variants. Circ Cardiovasc Genet 2014;7:677-83. [Crossref] [PubMed]
- Freeze SL, Landis BJ, Ware SM, et al. Bicuspid Aortic Valve: a Review with Recommendations for Genetic Counseling. J Genet Couns 2016;25:1171-8. [Crossref] [PubMed]
- Garg V, Muth AN, Ransom JF, et al. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270-4. [Crossref] [PubMed]
- Foffa I, Ait Ali L, Panesi P, et al. Sequencing of NOTCH1, GATA5, TGFBRI and TGFBRII genes in familial cases of bicuspid aortic valve. BMC Med Genet 2013;14:44. [Crossref] [PubMed]
- Girdauskas E, Schulz S, Borger MA, et al. Transforming growth factor-beta receptor type II mutation in a patient with bicuspid aortic valve disease and intraoperative aortic dissection. Ann Thorac Surg 2011;91:e70-e71. [Crossref] [PubMed]
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alphaactin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007;39:1488-93. [Crossref] [PubMed]
- Andelfinger G, Tapper AR, Welch RC, et al. KCNJ2 mutation results in Andersen syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am J Hum Genet 2002;71:663-8. [Crossref] [PubMed]
- Qu XK, Qiu XB, Yuan F, et al. A novel NKX2.5 loss-of-function mutation associated with congenital bicuspid aortic valve. Am J Cardiol 2014;114:1891-5. [Crossref] [PubMed]
- Pepe G, Nistri S, Giusti B, et al. Identification of fibrillin 1 gene mutations in patients with bicuspid aortic valve (BAV) without Marfan syndrome. BMC Med Genet 2014;15:23. [Crossref] [PubMed]
- Lee TC, Zhao YD, Courtman DW, et al. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000;101:2345-8. [Crossref] [PubMed]
- Padang R, Bagnall RD, Richmond DR, et al. Rare nonsynonymous variations in the transcriptional activation domains of GATA5 in bicuspid aortic valve disease. J Mol Cell Cardiol 2012;53:277-81. [Crossref] [PubMed]
- Foffa I, Murzi M, Mariani M, et al. Angiotensin-converting enzyme insertion/deletion polymorphism is a risk factor for thoracic aortic aneurysm in patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg 2012;144:390-5. [Crossref] [PubMed]
- Martín M, Pichel I, Flórez JP, et al. Low transcriptional activity haplotype of matrix metalloproteinase 1 is less frequent in bicuspid aortic valve patients. Gene 2013;524:304-8. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of American College of Cardiology/American heart Association task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for Aortic Dilatation in Patients With Bicuspid Aortic Valves: A statemet of clarification from the American College of Cardiology/American heart Association task Force on Practice Guidelines. J Am Coll Cardiol 2016;67:724-31. [Crossref] [PubMed]
- Braverman AC, Harris KM, Kovacs RJ, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 7: Aortic Diseases, Including Marfan Syndrome: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 2015;132:e303-9. [Crossref] [PubMed]
- Yuan SM. Bicuspid aortic valve in pregnancy. Taiwan J Obstet Gynecol 2014;53:476-80. [Crossref] [PubMed]
- Thornburg KL, Jacobson SL, Giraud GD, et al. Hemodynamic changes in pregnancy. Semin Perinatol 2000;24:11-4. [Crossref] [PubMed]
- Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC).ESC Committee for Practice Guidelines. Eur Heart J 2011;32:3147-97. [Crossref] [PubMed]
- McKellar SH, MacDonald RJ, Michelena HI, et al. Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. Am J Cardiol 2011;107:96-9. [Crossref] [PubMed]
- Della Corte A, Body SC, Booher AM, et al. Surgical treatment of bicuspid aortic valve disease: knowledge gaps and research perspectives. J Thorac Cardiovasc Surg 2014;147:1749-57. [Crossref] [PubMed]
- Goland S, Czer LS, De Robertis MA, et al. Risk factors associated with reoperation and mortality in 252 patients after aortic valve replacement for congenitally bicuspid aortic valve disease. Ann Thorac Surg 2007;83:931-7. [Crossref] [PubMed]
- Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32. [Crossref] [PubMed]
- Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000;36:1152-8. [Crossref] [PubMed]
- Arabkhani B, Mookhoek A, Di Centa I, et al. Reported Outcome After Valve-Sparing Aortic Root Replacement for Aortic Root Aneurysm: A Systematic Review and Meta-Analysis. Ann Thorac Surg 2015;100:1126-31. [Crossref] [PubMed]
- Badiu CC, Bleiziffer S, Eichinger WB, et al. Are bicuspid aortic valves a limitation for aortic valve repair? Eur J Cardiothorac Surg 2011;40:1097-104. [PubMed]
- Lausberg HF, Aicher D, Langer F, et al. Aortic valve repair with autologous pericardial patch. Eur J Cardiothorac Surg 2006;30:244-9. [Crossref] [PubMed]
- Aicher D, Schneider U, Schmied W, et al. Early results with annular support in reconstruction of the bicuspid aortic valve. J Thorac Cardiovasc Surg 2013;145:S30-4. [Crossref] [PubMed]
- Lansac E, Di Centa I, Sleilaty G, et al. Long-term results of external aortic ring annuloplasty for aortic valve repair. Eur J Cardiothorac Surg 2016;50:350-60. [Crossref] [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21. [PubMed]
- Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435-8. [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430-8. [Crossref] [PubMed]
- Furukawa H, Tanemoto K. Current topics on bicuspid aortic valve: clinical aspects and surgical management. Ann Thorac Cardiovasc Surg 2015;21:314-21. [Crossref] [PubMed]
- Yousef A, Simard T, Pourdjabbar A, et al. Performance of transcatheter aortic valve implantation in patients with bicuspid aortic valve: Systematic review. Int J Cardiol 2014;176:562-4. [Crossref] [PubMed]
- Mathieu P, Bossé Y, Huggins GS, et al. The pathology and pathobiology of bicuspid aortic valve: State of the art and novel research perspectives. J Pathol Clin Res 2015;1:195-206. [Crossref] [PubMed]


